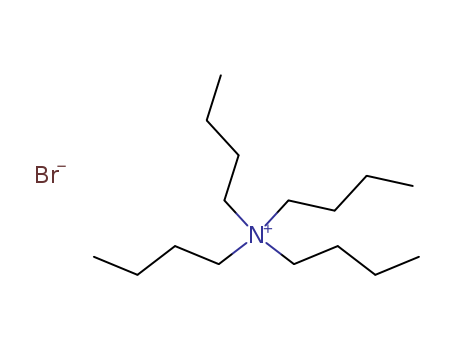
1643-19-2
- Product Name:Tetrabutylammonium bromide
- Molecular Formula:C16H36BrN
- Purity:99%
- Molecular Weight:322.373
Product Details;
CasNo: 1643-19-2
Molecular Formula: C16H36BrN
Appearance: white crystals or powder
Reputable Manufacturer Supply 1643-19-2 On Stock, Factory Sells Tetrabutylammonium bromide
- Molecular Formula:C16H36BrN
- Molecular Weight:322.373
- Appearance/Colour:white crystals or powder
- Vapor Pressure:0Pa at 25℃
- Melting Point:102-106 °C(lit.)
- Refractive Index:n20/D 1.422
- Boiling Point:102°C
- PKA:0[at 20 ℃]
- Flash Point:100℃
- PSA:0.00000
- Density:1.039 g/mL at 25 °C
- LogP:2.00760
Tetrabutylammonium bromide(Cas 1643-19-2) Usage
|
Chemical Composition and Structure |
Tetrabutylammonium bromide (TBAB) is a quaternary ammonium salt composed of four butyl groups attached to a central nitrogen atom, with a bromide anion. Its chemical structure allows it to act as a phase transfer catalyst and as a component in deep eutectic solvents (DESs). |
|
Mechanism of Action |
TBAB functions as a phase transfer catalyst, facilitating the transport of reactants between aqueous and organic phases. It can also act as a co-catalyst, enhancing reaction rates and product yields in various chemical processes. |
|
Uses |
TBAB is employed as a catalyst in reactions such as alkylation, oxidation, reduction, esterification, and coupling reactions. It is also used as a zwitterionic solvent under molten conditions and as a component in DESs for green chemistry applications. |
|
Production Methods |
TBAB can be synthesized through chemical processes involving the reaction of butyl bromide with tetra-n-butylammonium hydroxide. |
|
Physical and Chemical Properties |
Tetrabutylammonium bromide, also known as tetrabutylammonium bromide. White crystal, deliquescence. 118 ℃ melting point. Soluble in water, alcohol, ether and acetone, slightly soluble in benzene. Figure 1:? a structural formula of tetrabutylammonium bromide |
|
Application |
(1) Used as a reagent for the analysis of organic synthesis. (2) Tetrabutylammonium bromide is also an effective phase transfer catalyst. Phase transfer catalyst, referred to as PTC, is able to transfer the aqueous phase (or organic phase) to the organic phase (or aqueous phase) catalyst, which can make the reaction between the aqueous phase and the organic phase of the catalyst. PTC has the function of changing the degree of ion solvation, increasing the activity of ion reaction, speeding up the reaction rate and so on. Solve the problem of the past in the two phases of the reaction is difficult to react. Common quaternary ammonium salt phase transfer catalysts are: benzyl triethyl ammonium chloride, trioctyl methyl ammonium chloride, tetramethyl ammonium bromide, tetrapropylammonium chloride, tetrabutylammonium bromide , tetrabutyl ammonium iodide, benzyl triethyl ammonium bromide, triethyl hexyl bromide, octyl triethylammonium bromide. Phase transfer catalyst is widely applied in organic synthesis: R2C for preparing compounds (carbene type compound), further preparation of the corresponding nitrile, isonitrile, Halon, dichloromethane cyclopropane derivatives, hydroxy acids and diazomethane. For the alkylation reaction, compared with traditional methods, to avoid the harsh conditions of dry operation, and high yield, it can also be used in the redox reaction, ester hydrolysis, substitution reaction, condensation reaction, addition reaction, polymerization reaction, addition reaction of carbon and the elimination of reaction and so on. (3) For organic synthesis intermediates, phase transfer catalyst (4) Ion-pairing reagents for the synthesis of bacampicillin, sultamicillin like. (5) Ion pair chromatography reagents, phase transfer catalyst. Bacampicillin, sultamicillin like synthesis. |
|
preparation |
Preparative Methods: several methods are available to recover the quaternary ammonium ion efficiently.Prepared by reaction of tri-n-butylamine and n-butyl bromide. |
|
Toxicity |
The acute oral LD50 (mouse): 590mg/kg. Inhalation, ingestion and skin contact toxic to the skin, eyes and respiratory system irritation. More information from the lookchem Xiaonan editor (2015-09-16). |
|
Chemical Properties |
white crystals or powder |
|
Definition |
ChEBI: Tetrabutylammonium bromide is a tetrabutylammonium salt with bromide as the anionic counterpart. It is an organic bromide salt and a tetrabutylammonium salt. |
|
General Description |
Tetrabutylammonium bromide, a quaternary ammonium compound widely used as a phase transfer catalyst. TBAB decreases the retention time and removes peak tailing by acting as an ion pair reagent during the chromatographic analysis of quaternary ammonium compounds. In the molten state, TBAB behaves like an ionic liquid, which is a promising green alternative to organic solvents in polymer synthesis. |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise the salt from *benzene (5mL/g) at 80o by adding hot n-hexane (three volumes) and allowing to cool. Dry it over P2O5 or Mg(ClO4)2, under vacuum. The salt is very hygroscopic. It can also be crystallised from ethyl acetate or dry acetone by adding diethyl ether and dried in vacuo at 60o for 2 days. It has been crystallised from acetone by addition of diethyl ether. It is so hygroscopic that all manipulations should be carried out in a dry-box. It has been purified by precipitation from a saturated solution in dry CCl4 on addition of cyclohexane or by recrystallisation from ethyl acetate, then heating in vacuum to 75o in the presence of P2O5. [Symons et al. J Chem Soc, Faraday Trans 1 76 2251 1908.] It also recrystallises from CH2Cl2/diethyl ether and is dried in a vacuum desiccator over P2O5. [Blau & Espenson J Am Chem Soc 108 1962 1986, Beilstein 4 IV 657.] |
InChI:InChI=1/C16H36N.N3/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-3-2/h5-16H2,1-4H3;/q+1;-1
1643-19-2 Relevant articles
-
Buckles,Harris
, p. 886 (1957)
-
NMR study of the complex formation between tert-butyl hydroperoxide and tetraalkylammonium bromides
Turovskij, Nikolaj A.,Berestneva, Yulia V.,Raksha, Elena V.,Zubritskij, Mikhail Yu.,Grebenyuk, Serhiy A.
, p. 1443 - 1448 (2014)
The interaction between tert-butyl hydro...
Atom transfer radical addition (ATRA) catalyzed by copper complexes with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) ligand
Kaur, Aman,Gorse, Erin E.,Ribelli, Thomas G.,Jerman, Callista C.,Pintauer, Tomislav
, p. 246 - 252 (2015)
Synthesis, characterization, electrochem...
-
Sadek,Fuoss
, p. 301,305 (1950)
-
Structure and Stability of Quaternary Ammonium Interhalides: Experimental and Quantum-Chemical Study
Simonyan,Kletskii,Chernov'yants,Gol'eva
, p. 575 - 582 (2003)
The electronic structure of a series of ...
-
Lehmkuhl,H. et al.
, p. 41 - 52 (1973)
-
Alternative mechanistic scheme for salt effects on solvolysis reactions of haloalkanes and related compounds in binary DMSO/H2O solvent mixture
Hojo, Masashi,Aoki, Sho
, p. 1023 - 1030,8 (2012)
In 75% (v/v) DMSO/H2O solvent mixture, s...
Synthesis method of tetrabutylammonium bromide
-
Paragraph 0024-0029; 0030-0035; 0036-0041; 0042-0047; ..., (2021/08/14)
The invention discloses a synthesis meth...
Lewis Acidity Scale of Diaryliodonium Ions toward Oxygen, Nitrogen, and Halogen Lewis Bases
Legault, Claude Y.,Mayer, Robert J.,Mayr, Herbert,Ofial, Armin R.
supporting information, (2020/03/13)
Equilibrium constants for the associatio...
Synthesis process of tetrabutylammonium bromide
-
Paragraph 0061-0072, (2020/12/06)
The invention discloses a synthesis proc...
Application of ionic liquid in synthesis of propylene glycol ether and synthetic method of propylene glycol ether
-
Paragraph 0089; 0090, (2018/03/01)
The invention relates to the technical f...
1643-19-2 Process route
-
-
C16H36N(1+)*Br(1-)*C4H10O2

-
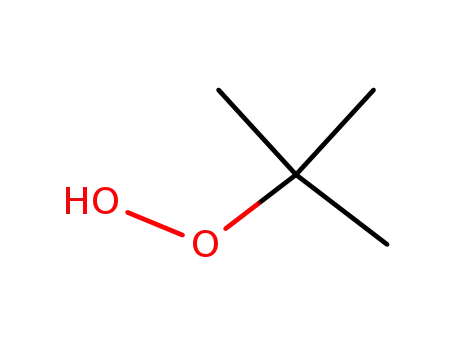
- 75-91-2
tert.-butylhydroperoxide

-
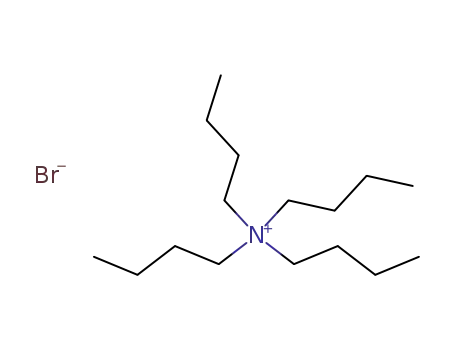
- 1643-19-2
tetrabutylammomium bromide
| Conditions | Yield |
|---|---|
|
In [D3]acetonitrile; at 24.84 ℃; Equilibrium constant;
|
-
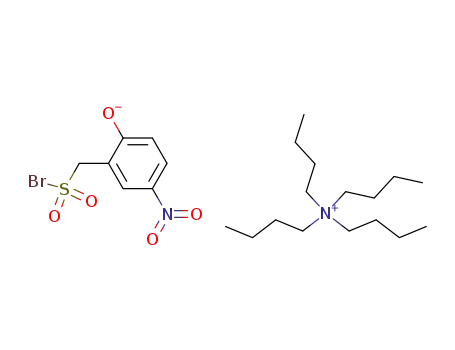
-
tetra(n-butyl)ammonium 2-hydroxy-5-nitrotoluene-α-sulphonyl bromide

-
- 14618-10-1
5-Nitrobenz<1,6-d>-3H-1,2-oxathiole S,S-dioxide

-
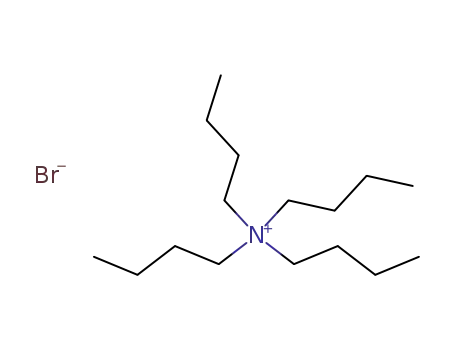
- 1643-19-2
tetrabutylammomium bromide
| Conditions | Yield |
|---|---|
|
In nitrobenzene; Equilibrium constant; other solvent;
|
1643-19-2 Upstream products
-
109-65-9
1-bromo-butane
-
102-82-9

tributyl-amine
-
1923-70-2
tetrabutylammonium perchlorate
-
636-70-4
triethylamine hydrobromide
1643-19-2 Downstream products
-
94516-67-3
4,5-bis-(benzoylseleno)-1,3-diselenole-2-selone
-
64269-61-0
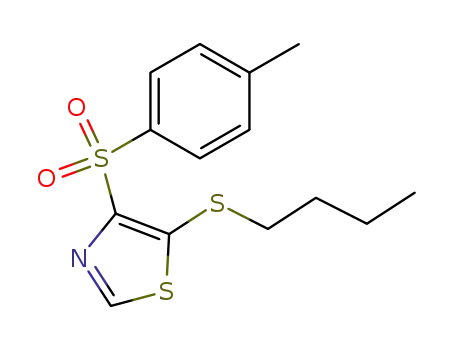
5-butylsulfanyl-4-(toluene-4-sulfonyl)-thiazole
-
64269-66-5
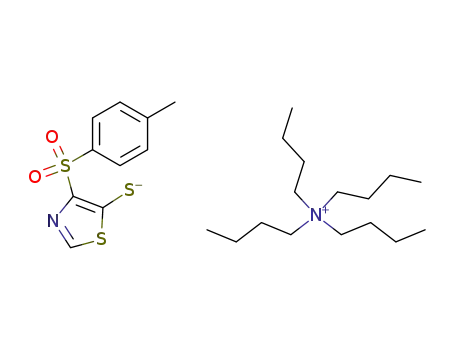
4-(toluene-4-sulfonyl)-4H-thiazole-5-thione; tetrabutylammonium salt
-
23231-91-6
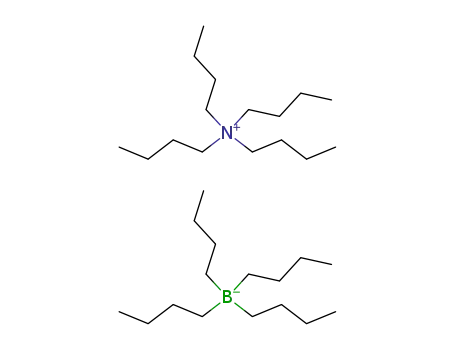
Tetrabutylammonium tetrabutylborate
Relevant Products
-
Scopolamine butylbromide
CAS:149-64-4
-
Acetamidine hydrochloride
CAS:124-42-5
-
N-Chlorosuccinimide
CAS:128-09-6