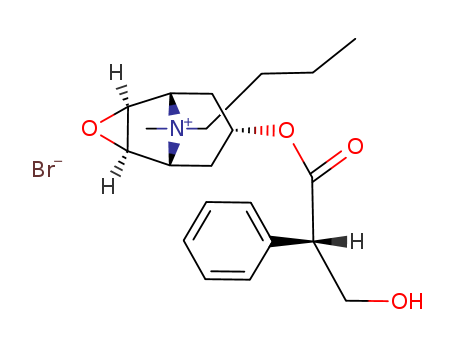
149-64-4
- Product Name:Scopolamine butylbromide
- Molecular Formula:C21H30NO4.Br
- Purity:99%
- Molecular Weight:440.377
Product Details;
CasNo: 149-64-4
Molecular Formula: C21H30NO4.Br
Appearance: White crystalline solid
Wholesale Trustworthy Manufacturer Supply Scopolamine butylbromide 149-64-4 In Stock
- Molecular Formula:C21H30NO4.Br
- Molecular Weight:440.377
- Appearance/Colour:White crystalline solid
- Melting Point:142-144 °C
- PSA:59.06000
- LogP:-0.80420
Scopolamine butylbromide(Cas 149-64-4) Usage
|
Chemical Properties |
Crystalline Solid |
|
Originator |
Butylscopolamine,China Pharm |
|
Uses |
Anticholinergic. Antispasmodic |
|
Manufacturing Process |
1300 g of scopolamine base and 350 g of n-butylbromide in 600 ml acetonitrile is heated at 65°C for 160 hours. The oil obtained is dissolved in methanol. The solution is cooled and crystalline scopolamine N-n-butylbromide is filtered. After recrystallization from methanol was obtained scopolamine N-n-butylbromide with melting point 142-144°C and [α]d 20 = -20.5° (3% solution in water); yield 65%. |
|
Therapeutic Function |
Anticholinergic, Spasmolytic, Antitussive |
|
Biochem/physiol Actions |
Competitive muscarinic acetylcholine receptor antagonist; antispasmodic. |
|
Clinical Use |
Symptomatic relief of gastrointestinal or genitourinary disorders due to smooth muscle spasm Bowel colic Excessive respiratory secretions |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
The main metabolic pathway is the hydrolytic cleavage of the ester bond. Orally administered hyoscine butylbromide is excreted in the faeces and in the urine. Studies in man show that 2-5% of radioactive doses is eliminated renally after oral, and 0.7-1.6% after rectal administration. Approximately 90% of recovered radioactivity can be found in the faeces after oral administration. The urinary excretion of hyoscine butylbromide is less than 0.1% of the dose. The metabolites excreted via the renal route bind poorly to muscarinic receptors and are therefore not considered to contribute to the effect of the hyoscine butylbromide. |
InChI:InChI=1/C21H30NO4.BrH/c1-3-4-10-22(2)17-11-15(12-18(22)20-19(17)26-20)25-21(24)16(13-23)14-8-6-5-7-9-14;/h5-9,15-20,23H,3-4,10-13H2,1-2H3;1H/q+1;/p-1/t15?,16-,17+,18+,19-,20+,22?;/m1./s1
149-64-4 Relevant articles
The synthesis of anticholinergically active N-alkylnorscopolamines and their quaternary salts with particular consideration of the bronchospasmolytic compound (-)-N-ethylnorscopolamine methobromide (Ba 253 BR)
Banholzer,Pook
, p. 217 - 228 (2007/10/02)
The synthesis of anticholinergic N-alkyl...
149-64-4 Process route
-
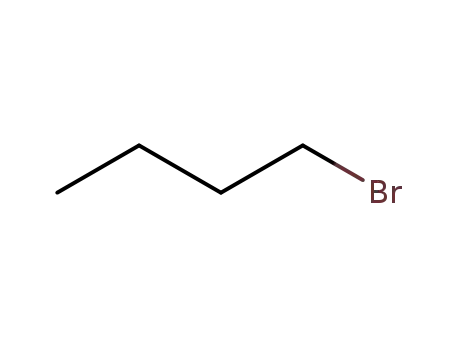
-
109-65-9
1-bromo-butane

-
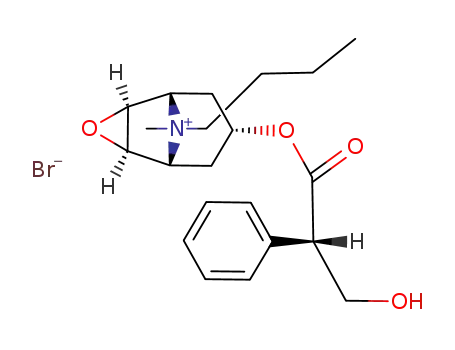
-
149-64-4
hyoscine-N-butyl bromide
| Conditions | Yield |
|---|---|
|
With
acetonitrile;
|
-
-
74-83-9
methyl bromide

-
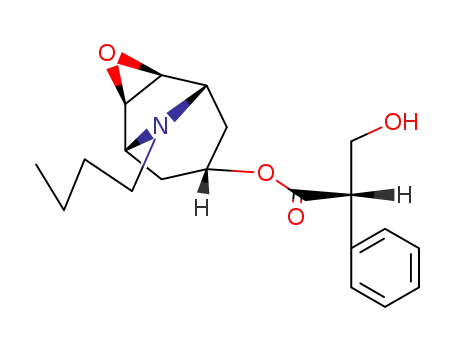
-
14861-14-4
N-butylscopolamine

-

-
149-64-4
hyoscine-N-butyl bromide
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
Ambient temperature;
|
149-64-4 Upstream products
-
74-83-9
methyl bromide
-
14861-14-4

N-butylscopolamine
-
109-65-9

1-bromo-butane
149-64-4 Downstream products
-
498-45-3
scopine
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
Nimodipine
CAS:66085-59-4
-
Leonurine
CAS:7097-09-8