124-42-5
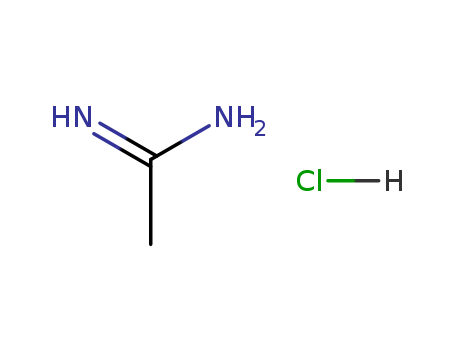
- Product Name:Acetamidine hydrochloride
- Molecular Formula:C2H6N2.HCl
- Purity:99%
- Molecular Weight:94.544
Product Details;
CasNo: 124-42-5
Molecular Formula: C2H6N2.HCl
Appearance: white fine crystalline powder
Reputable Factory Supply High Purity 99% Acetamidine hydrochloride 124-42-5 On Stock
- Molecular Formula:C2H6N2.HCl
- Molecular Weight:94.544
- Appearance/Colour:white fine crystalline powder
- Vapor Pressure:176mmHg at 25°C
- Melting Point:165-170 °C(lit.)
- Refractive Index:1.458
- Boiling Point:62.8 °C at 760 mmHg
- PKA:pK1: 1.60(+1) (25°C)
- Flash Point:26.1 °C
- PSA:49.87000
- Density:1.03g/cm3
- LogP:1.54430
Acetamidine hydrochloride(Cas 124-42-5) Usage
|
Chemical Description |
Acetamidine hydrochloride is an organic compound with a molecular formula C2H8ClN3. |
|
Uses |
Acetamidine Hydrochloride is a compound useful in organic synthesis. |
|
Chemical Properties |
WHITE FINE CRYSTALLINE POWDER |
|
General Description |
Acetamidine hydrochloride is an amidine salt and its conversion to 2,4,6-trimethyl-sym-triazine has been studied. |
|
Purification Methods |
The hydrochlorde can be recrystallised from small volumes of EtOH. Alternatively it is dissolved in EtOH, filtered, Et2O is added; filter the crystalline salt off under N2 and dry it in a vacuum desiccator over H2SO4. The salt is deliquescent and should be stored in a tightly stoppered container. Its solubility in H2O is 10% at room temperature and it is soluble in Me2CO. The free base reacts strongly alkaline in H2O. It has max 224nm ( 4000) in H2O. The picrate has m 252o (sintering at ~245o). [Dox Org Synth Coll Vol I 5 1941, Davies & Parsons Chem Ind (London) 628 1958, Barnes et al. J Am Chem Soc 62 1286 1940 give m 177-178o, Beilstein 2 H 185, 2 I 85, 2 II 183, 2 III 416, 2 IV 428.] |
InChI:InChI=1/C2H6N2/c1-2(3)4/h1H3,(H3,3,4)
124-42-5 Relevant articles
Preparation method of cefathiamidine hydrochloride
-
Paragraph 0039; 0062; 0066-0068; 0072-0074; 0078-0080; ..., (2021/09/01)
The invention provides a preparation met...
Synthesis, structures of some unsymmetrical 3,6-disubstituted-1,2,4,5- tetrazines
Hu, Wei-Xiao,Xu, Feng
scheme or table, p. 1745 - 1750 (2009/05/31)
(Chemical Equation Presented) A series o...
Pyrazole derivatives
-
, (2008/06/13)
This invention relates to pyrazole deriv...
124-42-5 Process route
-
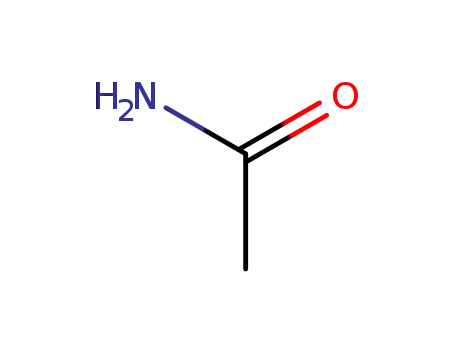
- 60-35-5
acetamide

-
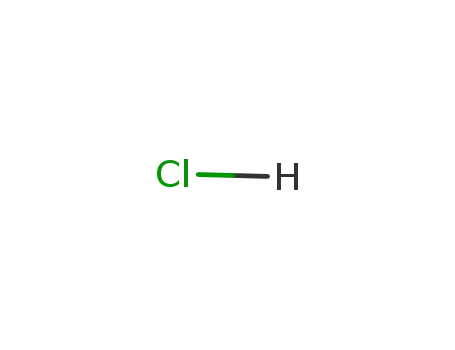
- 7647-01-0,15364-23-5
hydrogenchloride

-
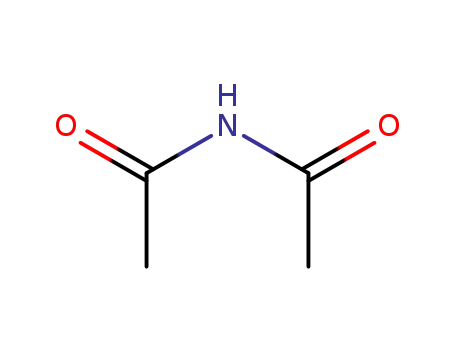
- 625-77-4
N-acetylacetamide

-
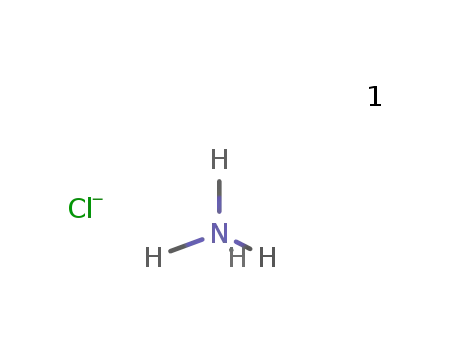
-
ammonium chloride

-
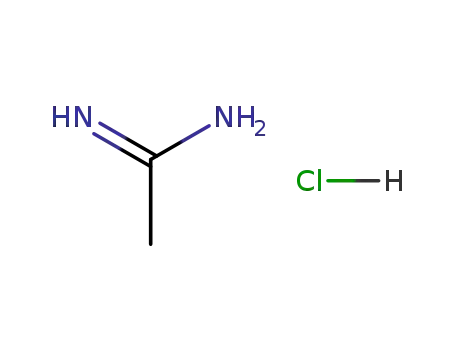
- 124-42-5
acetamidine hydrochloride

-
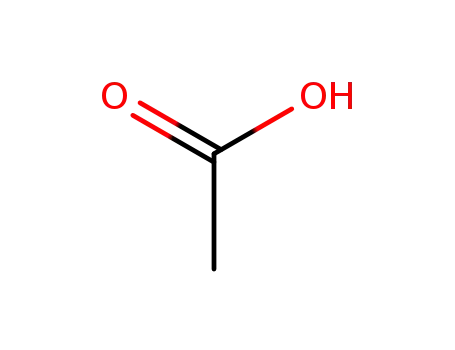
- 64-19-7,77671-22-8
acetic acid
| Conditions | Yield |
|---|---|
|
beim Destillieren;
|
-
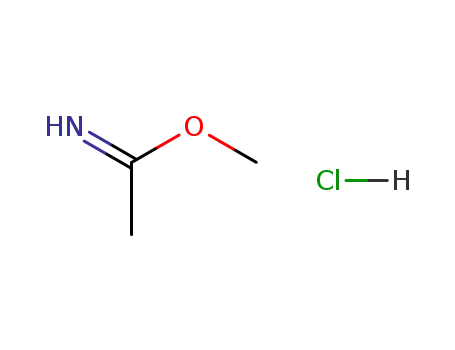
- 14777-27-6
methyl acetimidate hydrochloride

-

- 124-42-5
acetamidine hydrochloride
| Conditions | Yield |
|---|---|
|
With ammonia; In methanol; at 0 - 28 ℃; for 2h; Temperature;
|
97.5% |
124-42-5 Upstream products
-
5426-04-0
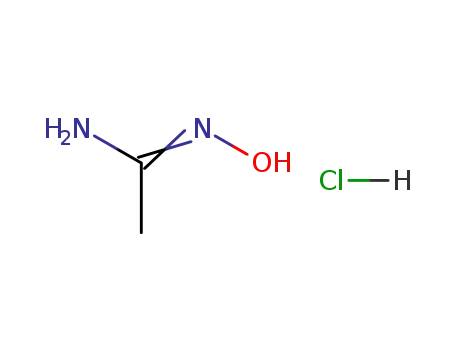
N-hydroxyacetamidine monohydrochloride
-
75-05-8

acetonitrile
-
1000-84-6
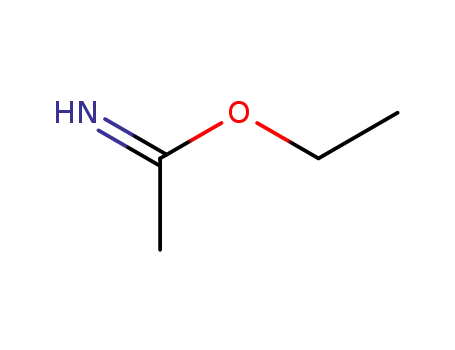
O-ethyl acetimidate
-
60-29-7
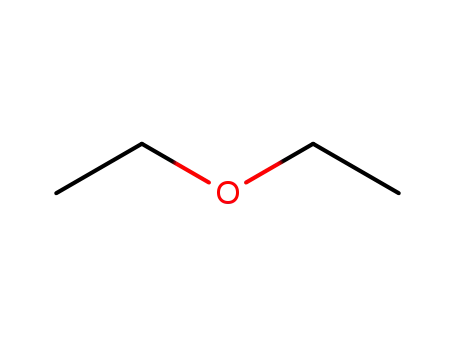
diethyl ether
124-42-5 Downstream products
-
6622-92-0
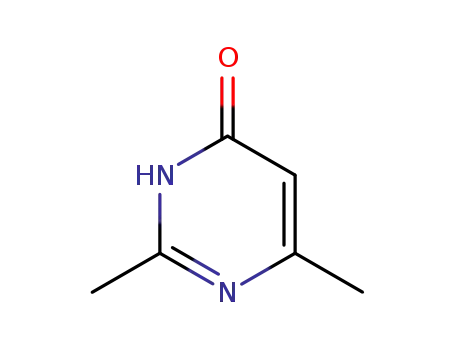
2,6-dimethyl-1H-pyrimidin-4-one
-
3599-87-9
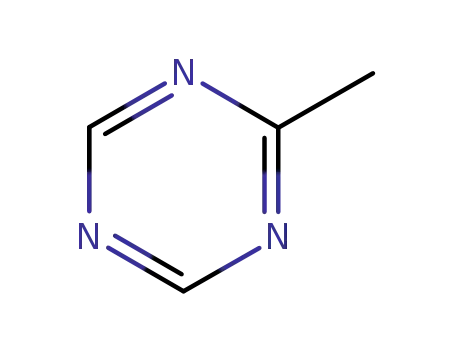
2-methyl-sym-triazine
-
65680-14-0
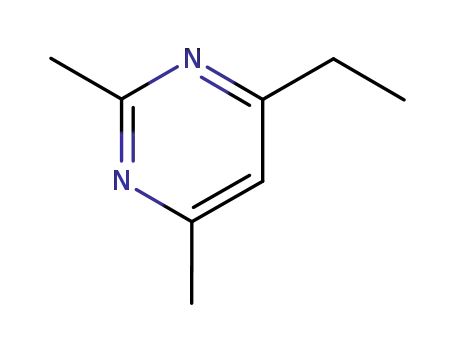
4-Athyl-2,6-dimethylpyrimidin
-
33663-57-9
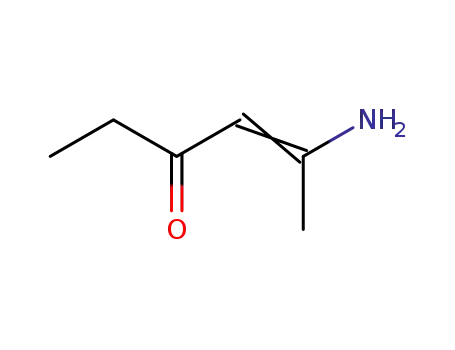
5-amino-4-hexen-3-one
Relevant Products
-
Benzathine Cloxacillin
CAS:23736-58-5
-
Acetazolamide
CAS:59-66-5
-
Tetrabutylammonium bromide
CAS:1643-19-2