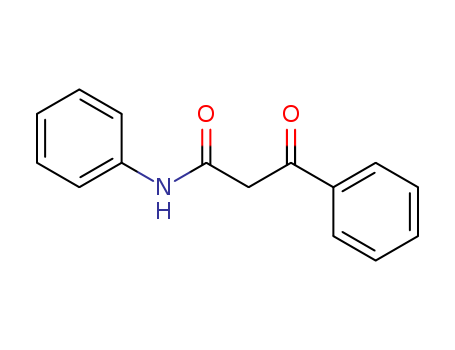
959-66-0
- Product Name:2-BENZOYLACETANILIDE
- Molecular Formula:C15H13NO2
- Purity:99%
- Molecular Weight:239.274
Product Details;
CasNo: 959-66-0
Molecular Formula: C15H13NO2
Appearance: light yellow powder
High Purity 99% Factory Supply 2-BENZOYLACETANILIDE 959-66-0 with Efficient Shipping
- Molecular Formula:C15H13NO2
- Molecular Weight:239.274
- Appearance/Colour:light yellow powder
- Vapor Pressure:3.9E-09mmHg at 25°C
- Melting Point:106-108 °C(lit.)
- Refractive Index:1.624
- Boiling Point:473.6°Cat760mmHg
- PKA:11.37±0.23(Predicted)
- Flash Point:194.5°C
- PSA:46.17000
- Density:1.205g/cm3
- LogP:2.97110
2-BENZOYLACETANILIDE(Cas 959-66-0) Usage
|
Chemical Properties |
light yellow powder |
|
General Description |
2-Benzoylacetanilide reacts with [PtCl2(cod)] (cod=cyclo-octa-1,5-diene), cis-[PtCl2(PPh3)2] (PPh3= triphenylphosphine) and [PdCl2(bipy)] (bipy=2,2′-bipyridine) to yield metallalactam complexes. |
InChI:InChI=1/C15H13NO2/c17-14(12-7-3-1-4-8-12)11-15(18)16-13-9-5-2-6-10-13/h1-10H,11H2,(H,16,18)
959-66-0 Relevant articles
Catalyst- And Additive-Free Approach to Constructing Benzo-oxazine, Benzo-oxazepine, and Benzo-oxazocine: O Atom Transfer and C-O, C-N, and C-O Bond Formation at Room Temperature
Ghosh, Arnab,Hegde, Rajeev V.,Rode, Haridas B.,Ambre, Ram,Mane, Manoj V.,Patil, Siddappa A.,Sridhar, Balasubramanian,Dateer, Ramesh B.
supporting information, p. 8189 - 8193 (2021/11/01)
An exclusive synthesis of benzo-oxazine,...
Ru-NHC-Catalyzed Asymmetric Hydrogenation of 2-Quinolones to Chiral 3,4-Dihydro-2-Quinolones
Daniliuc, Constantin,Glorius, Frank,Hu, Tianjiao,Lückemeier, Lukas
supporting information, p. 23193 - 23196 (2021/09/25)
Direct enantioselective hydrogenation of...
1,4,2-Dioxazol-5-ones as Isocyanate Equivalents: An Efficient Synthesis of 2-Quinolinones via β-Keto Amides
Vala, Anand,Parmar, Nirali,Soni, Jigar Y.,Kotturi, Sharadsrikar,Guduru, Ramakrishna
supporting information, p. 2080 - 2084 (2021/10/07)
Under thermal conditions, 1,4,2-dioxazol...
Palladium catalysed hydrolysis-free arylation of aliphatic nitriles for the synthesis of 4-arylquinolin-2-one/pyrazolone derivatives
Krishna Reddy, Singarajanahalli Mundarinti,Prasanna Kumari, Subramaniyan,Selva Ganesan, Subramaniapillai
, (2021/08/03)
Palladium catalysed addition of arylboro...
959-66-0 Process route
-
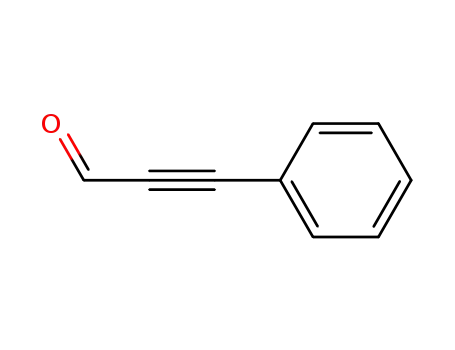
-
2579-22-8
Phenylpropargyl aldehyde

-
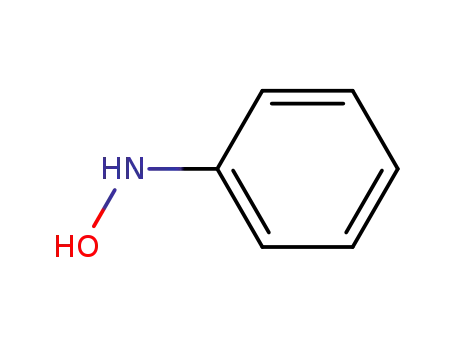
-
100-65-2
N-Phenylhydroxylamine

-
-
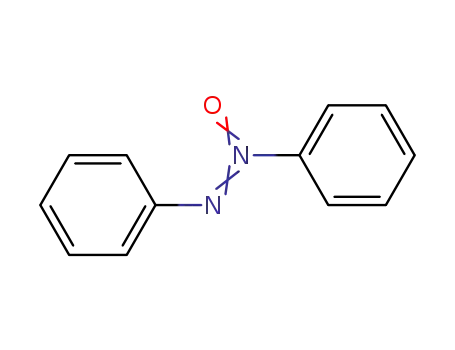
495-48-7,55599-32-1
azoxybenzene

-
-
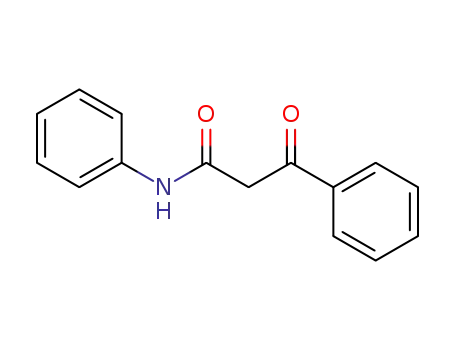
959-66-0
3-oxo-3-phenylpropionanilide
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
In
toluene;
at 40 ℃;
for 2h;
Inert atmosphere;
|
51% 8% |
|
With
water;
In
toluene;
at 40 ℃;
for 2h;
Inert atmosphere;
|
23% 4% |
-
-
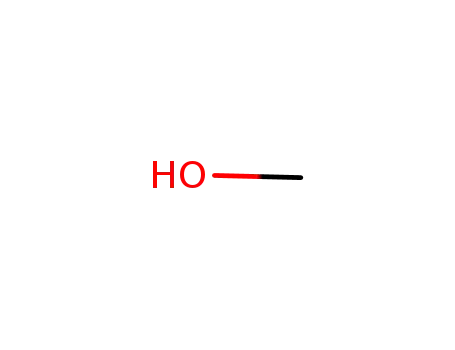
67-56-1
methanol

-
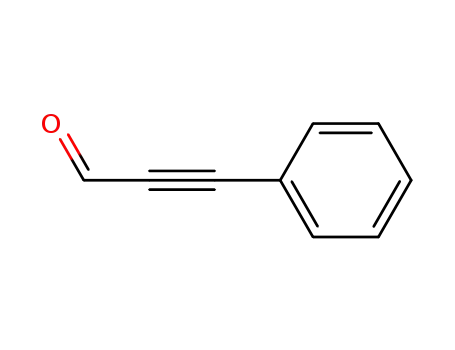
-
2579-22-8
Phenylpropargyl aldehyde

-

-
100-65-2
N-Phenylhydroxylamine

-
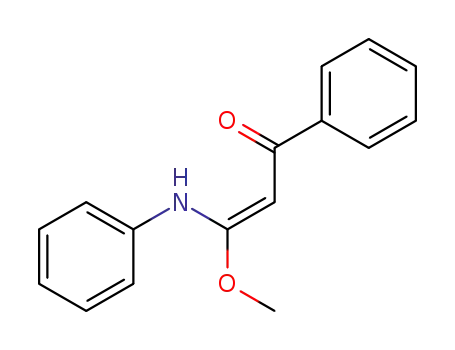
-
(E)-3-methoxy-1-phenyl-3-(phenylamino)prop-2-en-1-one

-

-
495-48-7,55599-32-1
azoxybenzene

-

-
959-66-0
3-oxo-3-phenylpropionanilide
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
at 25 ℃;
for 6h;
Solvent;
Molecular sieve;
Inert atmosphere;
|
91% |
959-66-0 Upstream products
-
64-17-5
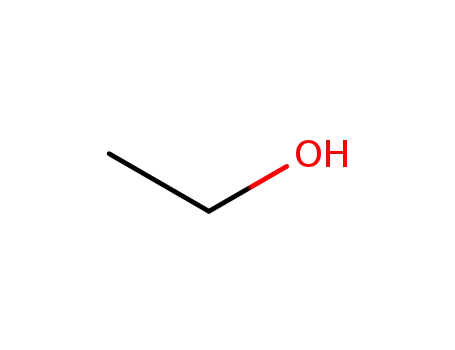
ethanol
-
52903-48-7
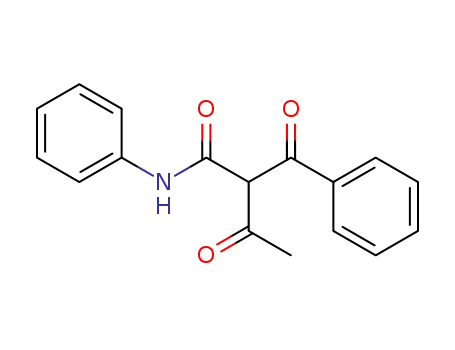
2-benzoyl-acetoacetic acid anilide
-
614-27-7
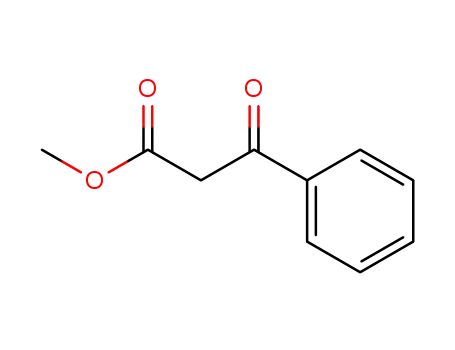
methyl 3-oxo-3-phenylpropionate
-
52561-86-1
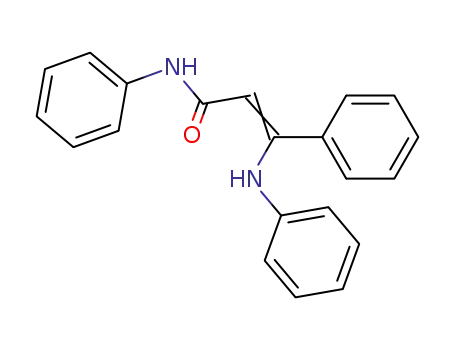
β-anilino-cinnamic acid anilide
959-66-0 Downstream products
-
25559-60-8
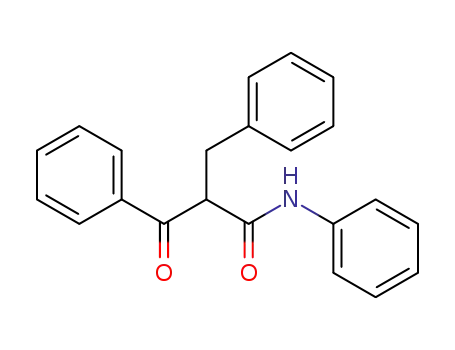
2-benzyl-3-oxo-3-phenyl-propionic acid anilide
-
742-29-0
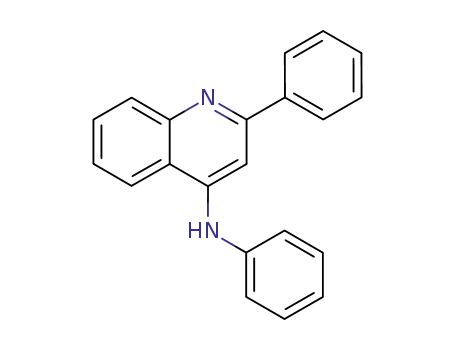
N-(2-phenyl-4-quinolyl)aniline
-
52561-84-9
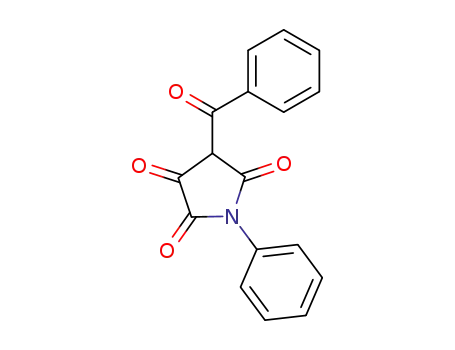
1-phenyl-4-benzoyl-pyrrolidine-2,3,5-trione
-
4754-92-1
2-(4-dimethylamino-phenylimino)-3-oxo-3-phenyl-propionic acid anilide
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
P-AMINOBENZAMIDE GLUTAMIC ACID
CAS:4271-30-1
-
Allopurinol
CAS:315-30-0