623-33-6
- Product Name:Glycine ethyl ester hydrochloride
- Molecular Formula:C4H10ClNO2
- Purity:99%
- Molecular Weight:139.582
Product Details;
CasNo: 623-33-6
Molecular Formula: C4H10ClNO2
Appearance: Crystalline
Quality Manufacturer Supply Top Purity Glycine ethyl ester hydrochloride 623-33-6 with Competitive Price
- Molecular Formula:C4H10ClNO2
- Molecular Weight:139.582
- Appearance/Colour:Crystalline
- Vapor Pressure:24.7mmHg at 25°C
- Melting Point:145-146 °C(lit.)
- Boiling Point:109.5 °C at 760 mmHg
- PKA:pK1:7.66(+1) (25°C)
- PSA:52.32000
- Density:1 g/cm3
- LogP:1.01050
Glycine ethyl ester hydrochloride(Cas 623-33-6) Usage
|
Chemical Properties |
Crystalline |
|
Uses |
As a Glycine (G615990) ester, Glycine ethyl ester hydrochloride can be used as parakeratosis inhibitor and external composition for skin. |
|
Synthesis |
A production method for glycine ethyl ester hydrochloride:First add dehydrated ethanol in 1# reactor, pass into anhydrous hydrogen chloride to obtain hydrochloric acid-ethanol solution; Add a certain amount of triethyl orthoformate and glycine and hydrochloric acid-ethanol solution in 2# reactor, in the Reaction under action; after the reaction is complete, distill and recover ethanol and ethyl formate; then add alcohol-ether mixture, wash, filter, concentrate, recrystallize, and vacuum dry to obtain ethyl glycine hydrochloride. |
|
Purification Methods |
Crystallise it from absolute EtOH or EtOH/Et2O. [Marvel Org Synth Coll Vol II 310 1943, Beilstein 4 II 780, 4 III 3 75.] |
InChI:InChI=1/C4H9NO2/c1-2-7-4(6)3-5/h2-3,5H2,1H3/p+1
623-33-6 Relevant articles
Construction and Evaluation of Molecular Models: Guide and Design of Novel SE Inhibitors
An, Yunfei,Dong, Yue,Han, Jun,Min, Liu,Sun, Bin,Zhao, Dongmei,Zhao, Liyu
, p. 1152 - 1159 (2020)
Squalene epoxidase (SE) was considered a...
Synthesis of deuterium and C-13-labelled ethyl glycolate and their subsequent use in the synthesis of labelled analogues of the DNA adduct O 6-carboxymethyl-2′-deoxyguanosine
Moore, Sharon A.,Shuker, David E. G.
, p. 855 - 858 (2011)
The adduct O6-carboxymethyl-2′-deoxyguan...
Dispirooxindoles based on 2-selenoxo-imidazolidin-4-ones: Synthesis, cytotoxicity and ros generation ability
Novotortsev, Vladimir K.,Kukushkin, Maxim E.,Tafeenko, Viktor A.,Skvortsov, Dmitry A.,Kalinina, Marina A.,Timoshenko, Roman V.,Chmelyuk, Nelly S.,Vasilyeva, Liliya A.,Tarasevich, Boris N.,Gorelkin, Petr V.,Erofeev, Alexander S.,Majouga, Alexander G.,Zyk, Nikolai V.,Beloglazkina, Elena K.
, p. 1 - 26 (2021/03/09)
A regio-and diastereoselective synthesis...
Novel naphthylamide derivatives as dual-target antifungal inhibitors: Design, synthesis and biological evaluation
An, Yunfei,Dong, Yue,Liu, Min,Han, Jun,Zhao, Liyu,Sun, Bin
, (2020/11/13)
Fungal infections have become a serious ...
Structural Fine-Tuning of Desmuramylpeptide NOD2 Agonists Defines Their in Vivo Adjuvant Activity
Guzelj, Samo,Nabergoj, Sanja,Gobec, Martina,Pajk, Stane,Klan?i?, Veronika,Slütter, Bram,Frkanec, Ru?a,?timac, Adela,?ket, Primo?,Plavec, Janez,Mlinari?-Ra??an, Irena,Jakopin, ?iga
supporting information, p. 7809 - 7838 (2021/06/28)
We report on the design, synthesis, and ...
Eco-friendly synthesis of peptides using fmoc-amino acid chlorides as coupling agent under biphasic condition
Kantharaju, Kamanna,Khatavi, Santosh Y.
, p. 699 - 707 (2021/08/23)
Background: Agro-waste derived solvent m...
623-33-6 Process route
-
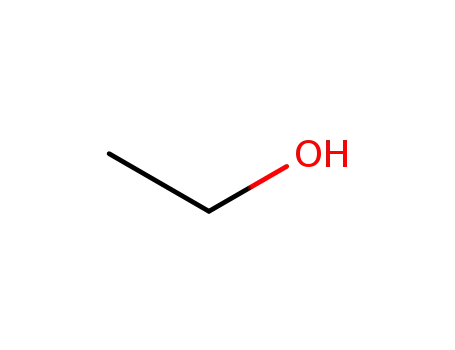
- 64-17-5
ethanol

-
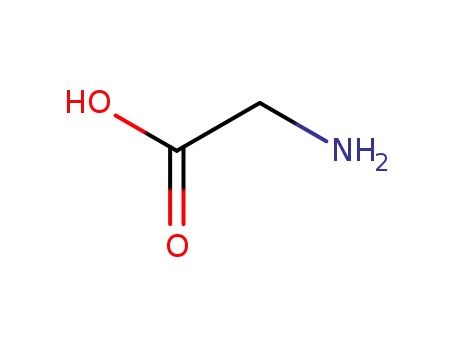
- 56-40-6,18875-39-3,25718-94-9
glycine

-
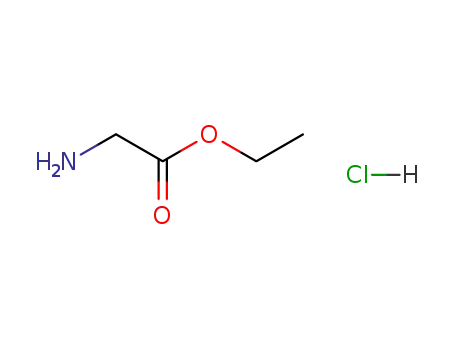
- 623-33-6
glycine ethyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
With thionyl chloride; at 25 - 30 ℃; for 0.583333h; microwave irradiation;
|
98% |
|
With thionyl chloride; Reflux;
|
97% |
|
With thionyl chloride; at -10 - 20 ℃; for 2h; Heating / reflux;
|
90.4% |
|
With thionyl chloride; Reflux;
|
90.48% |
|
With thionyl chloride; In ethanol; at 0 ℃; for 5h; Reflux;
|
90% |
|
With thionyl chloride; for 12h; Reflux;
|
88% |
|
ethanol; With acetyl chloride; at -5 - 5 ℃; for 1h;
glycine; at 70 ℃; for 5h;
|
88.98% |
|
With thionyl chloride; at 0 - 20 ℃; for 16h;
|
84% |
|
With thionyl chloride; at -15 ℃; for 1.41667h; Reflux;
|
48% |
|
With thionyl chloride; for 1.5h; Heating;
|
|
|
With hydrogenchloride; at 0 ℃; for 0.5h;
|
|
|
With thionyl chloride; at -5 - 78 ℃; for 1.5h; Reflux;
|
|
|
With thionyl chloride; at 78 ℃;
|
|
|
With acetyl chloride; at 85 ℃; for 0.5h; Inert atmosphere;
|
1.01 g |
|
With hydrogenchloride; at 20 ℃;
|
|
|
With thionyl chloride; for 2.5h; Reflux;
|
|
|
With thionyl chloride; at 78 ℃;
|
|
|
With thionyl chloride;
|
|
|
ethanol; With thionyl chloride; at 0 ℃; for 0.166667h;
glycine; at 70 ℃; for 3h;
|
|
|
ethanol; With thionyl chloride; at -10 - 0 ℃; for 1h;
glycine; at -10 ℃; for 7h; Reflux;
|
|
|
ethanol; With thionyl chloride; at 0 - 25 ℃;
glycine; at 25 ℃; Reagent/catalyst;
|
33.4 g |
|
With thionyl chloride; at 0 - 20 ℃;
|
|
|
With thionyl chloride; at 0 - 20 ℃; for 4h;
|
|
|
With thionyl chloride; at 0 ℃;
|
|
|
With thionyl chloride; at 0 ℃; for 5h; Reflux;
|
|
|
With thionyl chloride; at 0 - 65 ℃; for 6h;
|
|
|
With thionyl chloride; Reflux;
|
|
|
With thionyl chloride; Reflux;
|
|
|
With thionyl chloride; Reflux;
|
|
|
With thionyl chloride; at -10 - 20 ℃; for 0.6h;
|
-

- 56-40-6,18875-39-3,25718-94-9
glycine

-

- 623-33-6
glycine ethyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
With thionyl chloride; In ethanol; at -10 ℃; for 2h; Heating / reflux;
|
90.4% |
|
With thionyl chloride; In ethanol; for 2h;
|
90.4% |
|
|
623-33-6 Upstream products
-
64-17-5

ethanol
-
144232-41-7
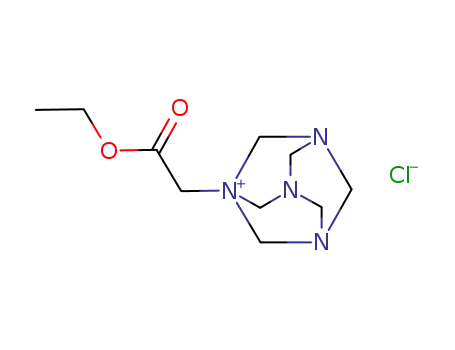
ethoxycarbonylmethyl-hexamethylenetetraminium; chloride
-
2184-96-5
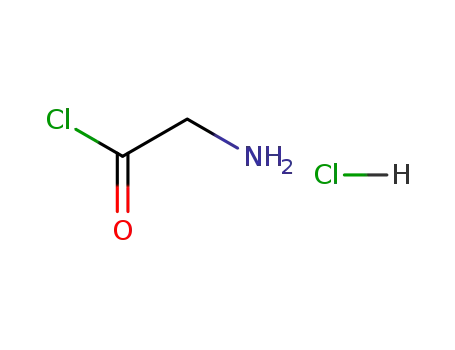
glycinyl chloride hydrochloride
-
56-40-6

glycine
623-33-6 Downstream products
-
4172-36-5
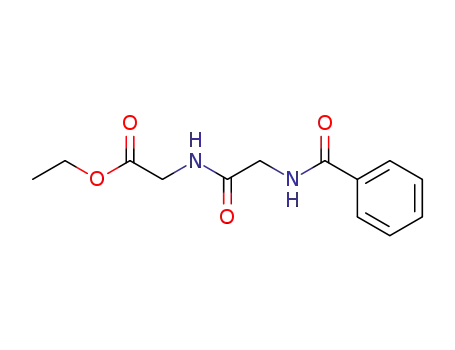
N-[(benzoylamino)acetyl]glycine ethyl ester
-
14181-05-6
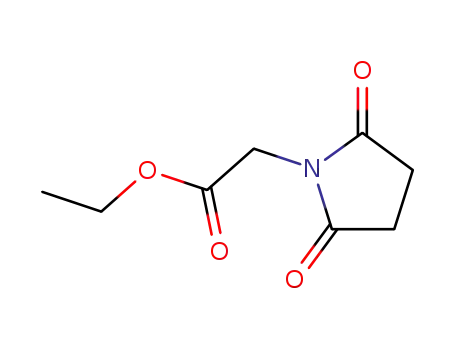
1-ethoxycarbonylmethyl-pyrrolidine-2,5-dione
-
101275-74-5
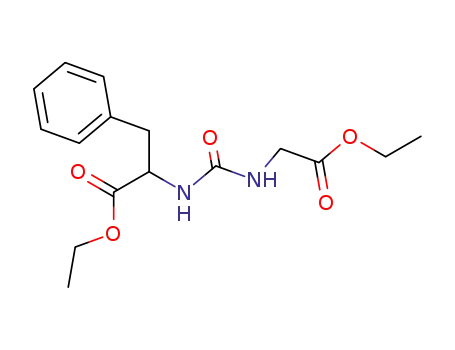
N-(ethoxycarbonylmethyl-carbamoyl)-phenylalanine ethyl ester
-
2949-22-6
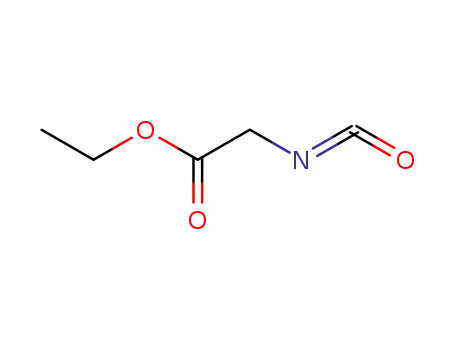
Glycine ethyl ester isocyanate
Relevant Products
-
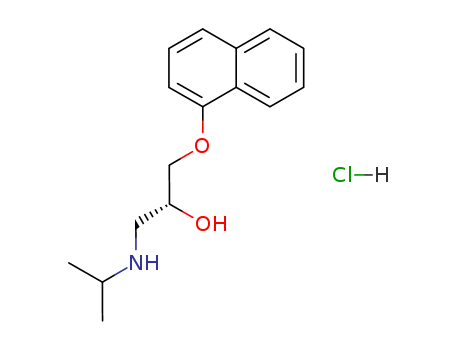
Propranolol Hydrochloride
CAS:3506-09-0
-
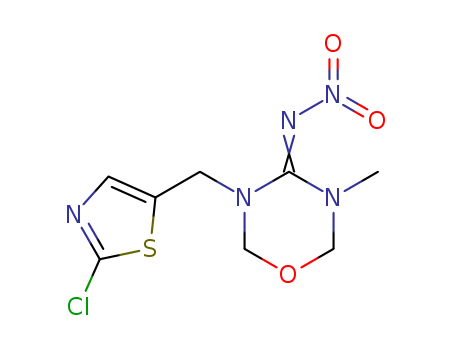
Thiamethoxam
CAS:153719-23-4
-
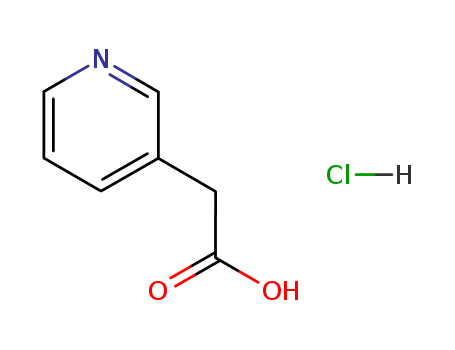
3-Pyridylacetic acid hydrochloride
CAS:6419-36-9