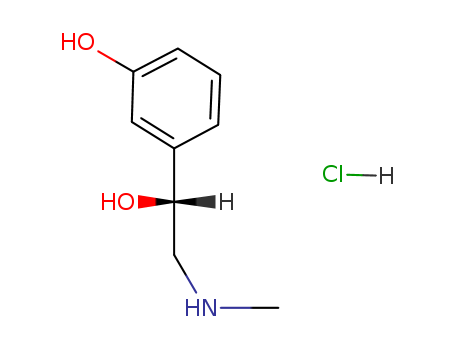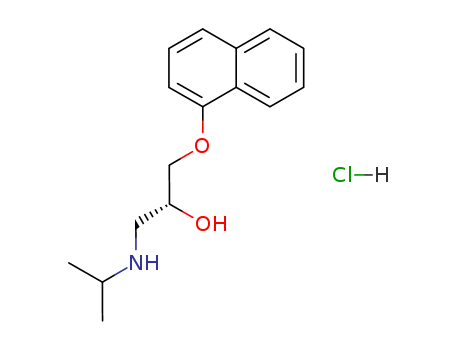
3506-09-0
- Product Name:Propranolol Hydrochloride
- Molecular Formula:C16H22ClNO2
- Purity:99%
- Molecular Weight:295.809
Product Details;
CasNo: 3506-09-0
Molecular Formula: C16H22ClNO2
Appearance: white powder
Buy Quality Reputable Factory Supply Propranolol Hydrochloride 3506-09-0 with Low Price
- Molecular Formula:C16H21NO2*ClH
- Molecular Weight:295.809
- Appearance/Colour:white powder
- Vapor Pressure:9.66E-09mmHg at 25°C
- Melting Point:163-165 °C(lit.)
- Boiling Point:446.1oC at 760 mmHg
- Flash Point:223.6oC
- PSA:41.49000
- LogP:3.77040
PROPRANOLOL HYDROCHLORIDE(Cas 3506-09-0) Usage
|
Uses |
Adrenoceptor antagonist, anti-arrythmic |
|
Biological Activity |
β -adrenergic antagonist. See separate isomers ((R)-(+)-Propranolol hydrochloride and (S)-(-)-Propranolol hydrochloride). |
InChI:InChI=1/C17H23NO2.ClH/c1-13(2)10-18-11-15(19)12-20-17-9-5-7-14-6-3-4-8-16(14)17;/h3-9,13,15,18-19H,10-12H2,1-2H3;1H
3506-09-0 Relevant articles
Covalent Organic Frameworks with Chirality Enriched by Biomolecules for Efficient Chiral Separation
Zhang, Sainan,Zheng, Yunlong,An, Hongde,Aguila, Briana,Yang, Cheng-Xiong,Dong, Yueyue,Xie, Wei,Cheng, Peng,Zhang, Zhenjie,Chen, Yao,Ma, Shengqian
supporting information, p. 16754 - 16759 (2018/11/27)
The separation of racemic compounds is i...
Ultrafast chiral separations for high throughput enantiopurity analysis
Barhate, Chandan L.,Joyce, Leo A.,Makarov, Alexey A.,Zawatzky, Kerstin,Bernardoni, Frank,Schafer, Wes A.,Armstrong, Daniel W.,Welch, Christopher J.,Regalado, Erik L.
supporting information, p. 509 - 512 (2017/01/13)
Recent developments in fast chromatograp...
Establishment and Evaluation of the Novel Tetramethylammonium-L-Hydroxyproline Chiral Ionic Liquid Synergistic System Based on Clindamycin Phosphate for Enantioseparation by Capillary Electrophoresis
Xu, Guangfu,Du, Yingxiang,Du, Fan,Chen, Jiaquan,Yu, Tao,Zhang, Qi,Zhang, Jinjing,Du, Shuaijing,Feng, Zijie
supporting information, p. 598 - 604 (2015/08/25)
Much attention has been paid to chiral i...
Uridine, thymidine and inosine used as chiral stationary phases in HPLC
Zhang, Mei,Zi, Min,Wang, Bang-Jin,Yuan, Li-Ming
, p. 2226 - 2228 (2014/06/09)
In this paper, we present the first enan...
3506-09-0 Process route
-
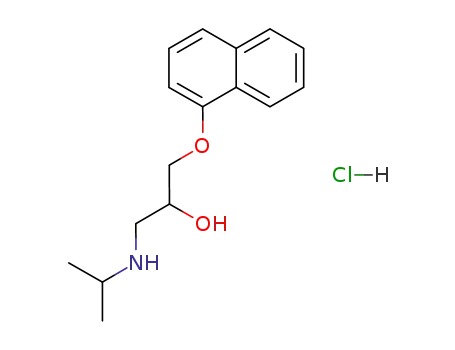
-
318-98-9
Inderal

-
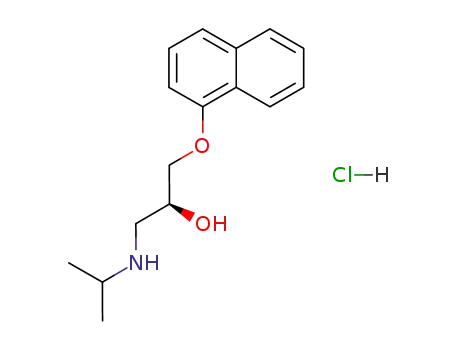
-
4199-10-4
(S)-(-)-propanolol hydrochloride

-
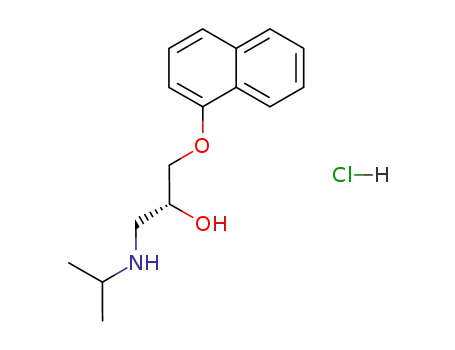
-
318-98-9,3506-09-0,4199-10-4,13071-11-9
(R)-propranolol hydrochloride
| Conditions | Yield |
|---|---|
|
With
sodium dihydrogenphosphate; phosphoric acid;
In
methanol; water;
pH=2.75;
Reagent/catalyst;
Ionic liquid;
Resolution of racemate;
|
|
|
With
uridine bonded to silica gel;
In
hexane; isopropyl alcohol;
at 30 ℃;
Resolution of racemate;
|
|
|
With
tetramethylammonium hydroxyproline; clindamycin phosphate;
In
methanol; water;
at 20 ℃;
pH=7.6;
pH-value;
Solvent;
Temperature;
enantioselective reaction;
Resolution of racemate;
|
|
|
With
carbon dioxide; isobutylamine;
In
methanol;
at 40 ℃;
under 150015 Torr;
Reagent/catalyst;
Solvent;
enantioselective reaction;
Resolution of racemate;
|
-
-
<2R-(2α(R*),3aα,4α,7α,7aα)>-N-(1-Methylethyl)-3-(1-naphthoxy)-2-<(octahydro-7,8,8-trimethyl-4,7-methanobenzofuran-2-yl)oxy>-1-propanamin

-

-
318-98-9,3506-09-0,4199-10-4,13071-11-9
(R)-propranolol hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol; diethyl ether;
|
89% |
3506-09-0 Upstream products
-
75-31-0
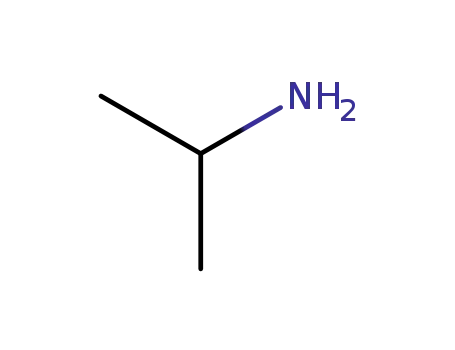
isopropylamine
-
132005-35-7
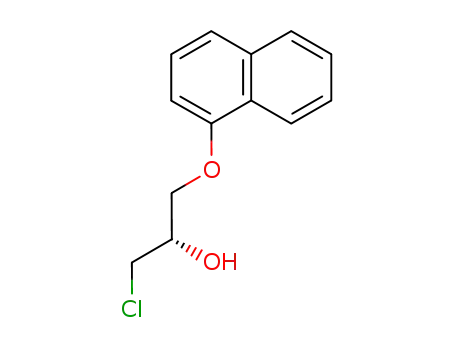
(S)-(+)-1-chloro-3-(1-naphthyloxy)-2-propanol
-
2461-42-9
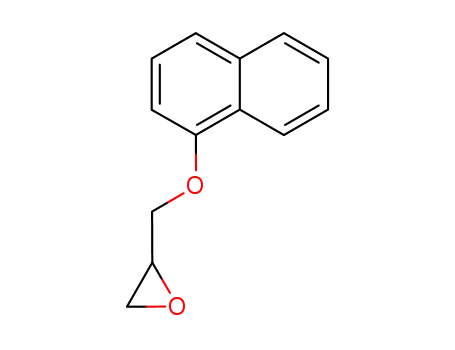
3-(1-naphthyloxy)-1,2-epoxypropane
-
90-15-3
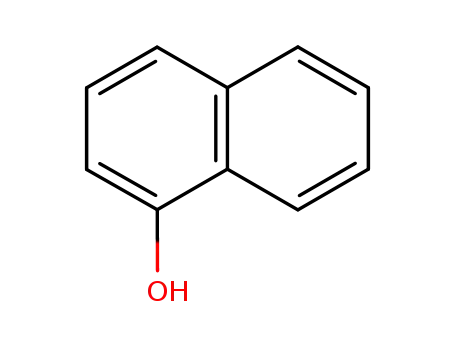
α-naphthol
3506-09-0 Downstream products
-
227176-57-0
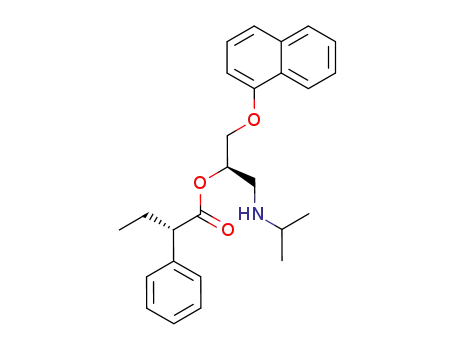
(S)-2-Phenyl-butyric acid (R)-1-(isopropylamino-methyl)-2-(naphthalen-1-yloxy)-ethyl ester
-
227176-55-8
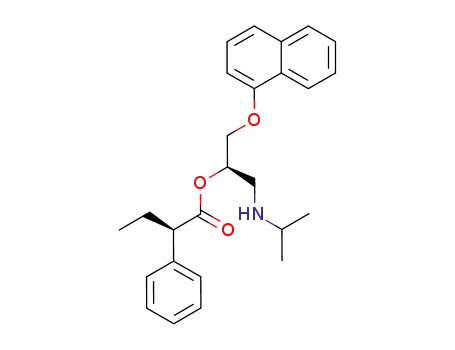
(R)-2-Phenyl-butyric acid (R)-1-(isopropylamino-methyl)-2-(naphthalen-1-yloxy)-ethyl ester
-
91796-22-4
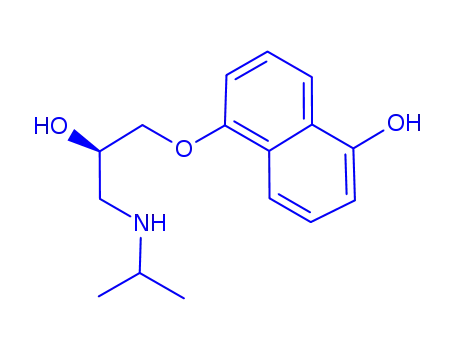
(+)-5-Hydroxypropranolol
-
393828-92-7
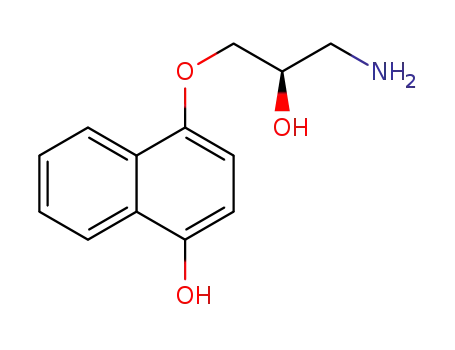
(R)-N-desisopropylpropranolol
Relevant Products
-
(R)-Phenylephrine Hydrochlorid
CAS:61-76-7
-
Epinephrine bitartrate
CAS:51-42-3
-
2-Deoxy-D-ribose
CAS:533-67-5