153719-23-4
- Product Name:Thiamethoxam
- Molecular Formula:C8H10ClN5O3S
- Purity:99%
- Molecular Weight:291.718
Product Details;
CasNo: 153719-23-4
Molecular Formula: C8H10ClN5O3S
Top Purity 99% Quality Manufacturer Supply Thiamethoxam 153719-23-4 with Efficient Transportation
- Molecular Formula:C8H10ClN5O3S
- Molecular Weight:291.718
- Vapor Pressure:1.36E-09mmHg at 25°C
- Melting Point:139.1°
- Refractive Index:1.725
- Boiling Point:485.8 ºC at 760 mmHg
- PKA:0.99±0.10(Predicted)
- Flash Point:247.6 ºC
- PSA:115.02000
- Density:1.71 g/cm3
- LogP:1.42220
Thiamethoxam(Cas 153719-23-4) Usage
|
Chemical Properties |
Off-White to Pale Yellow Solid |
|
Uses |
Thiamethoxam is a broad spectrum insecticide active against a wide spectrum of sucking and chewing insect pests after foliar, soil or seed treatment. |
|
Definition |
ChEBI: Thiamethoxam is an oxadiazane that is tetrahydro-N-nitro-4H-1,3,5-oxadiazin-4-imine bearing (2-chloro-1,3-thiazol-5-yl)methyl and methyl substituents at positions 3 and 5 respectively. It has a role as an antifeedant, a carcinogenic agent, an environmental contaminant, a xenobiotic and a neonicotinoid insectide. It is an oxadiazane, a member of 1,3-thiazoles, an organochlorine compound and a 2-nitroguanidine derivative. It derives from a 2-chlorothiazole. |
|
Application |
Thiamethoxam is a neonicotinoid insecticide that is used widely in Wisconsin. Thiamethoxam is the active ingredient in a variety of products used in agriculture to kill sucking and chewing insects that feed on roots, leaves, and other plant tissues. Agricultural uses include soil and seed treatments as well as leaf spraying for most row and vegetable crops like corn, soybeans, snap beans, and potatoes. It is also used to control insects in livestock pens, poultry houses, sod farms, golf courses, lawns, household plants, and tree nurseries. It was first registered by the U.S. Environmental Protection Agency in 1999. Reports show that when exposed to neonicotinid pesticides honeybees have probelms returnign home after foraging and bumblebee colonies grow poorly and produce fewer queens. |
|
Flammability and Explosibility |
Flammable |
|
Metabolic pathway |
All the information on thiamethoxam is taken from a summary of the proceedings of meeting published by the manufacturer. Full experimental details are not given in the report and the identity of metabolites is not disclosed (Novartis, 1997). |
|
Degradation |
Thiamethoxam is hydrolytically stable at pH 5 (half-life about 200-300 days). The compound is more labile at pH 9 where the half-life is a few days. It is rapidly photodegraded with a half-life of about 1 hour. In aquatic systems, degradation occurs under alkaline conditions and the insecticide is rapidly photodegraded but not readily biodegraded (Novartis, 1997). |
|
Mode of action |
Thiamethoxam interferes with nicotinic acetylcholine receptors in the insect’s nervous system, which are essential for proper functioning of the nerves. Within hours of contact or ingestion of thiamethoxam, insects stop feeding. Death usually occurs within 24 to 48 hours. The activity of thiamethoxam, or any insecticide, at the target site is just one factor in its efficacy. When comparing insecticides, other variables – including how the compound interacts with the environment, the plant and the insect – also contribute to its insecticidal mode of action. The Insecticide Resistance Action Committee (IRAC) has organized insecticides into 28 groups, plus sub-groups, based on their modes of action. Thiamethoxam is a Group 4A insecticide (neonicotinoids). |
InChI:InChI=1/C8H10ClN5O3S/c1-12-4-17-5-13(8(12)11-14(15)16)3-6-2-10-7(9)18-6/h2H,3-5H2,1H3/b11-8+
153719-23-4 Relevant articles
Experimental and DFT studies on the vibrational, electronic spectra and NBO analysis of thiamethoxam
Zhang, Fang,Zhang, Yu,Ni, Haiwei,Ma, Kuirong,Li, Rongqing
, p. 162 - 171 (2014)
Vibrational and electronic spectral meas...
Thiamethoxam production method and extractant
-
Paragraph 0088-0089; 0098-0099, (2020/05/01)
The invention provides a thiamethoxam pr...
Thiamethoxam and uses thereof
-
Paragraph 0085-0095, (2020/02/17)
A crystalline form of 3-(2-chloro-1,3-th...
Preparation method of thiamethoxam
-
Paragraph 0006; 0049-0068, (2019/10/01)
The invention discloses a preparation me...
PROCESS FOR THE PREPARATION OF THIAMETHOXAM
-
Page/Page column 18, (2019/05/22)
There is disclosed the use of a solvent ...
153719-23-4 Process route
-
-
105827-91-6
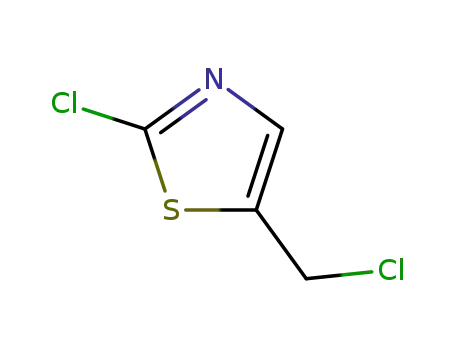
α-Cl-β-thiazolyl chloride

-
-
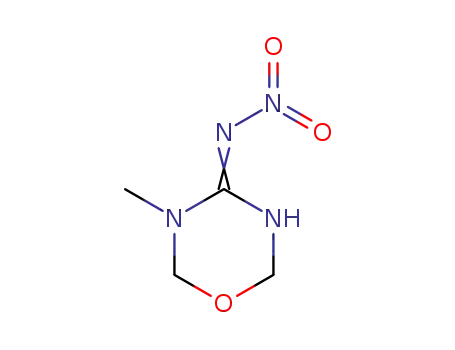
153719-38-1
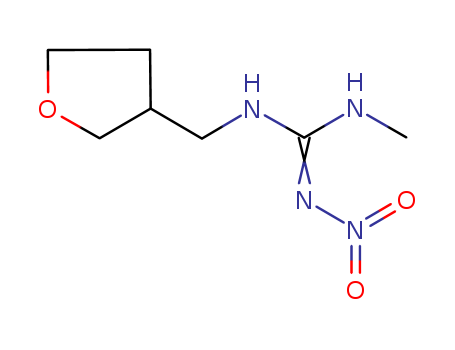
3,6-dihydro-3-methyl-N-nitro-2H-1,3,5-oxadiazin-4-amine

-
-
153719-23-4
thiamethoxam
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 50 ℃;
for 16h;
|
98% |
|
3,6-dihydro-3-methyl-N-nitro-2H-1,3,5-oxadiazin-4-amine;
With
potassium carbonate;
In
ethanol;
for 0.5h;
Autoclave;
α-Cl-β-thiazolyl chloride;
In
ethanol;
at 120 ℃;
for 4h;
Temperature;
Autoclave;
|
95.2% |
|
With
N-ethyl-N,N-diisopropylamine; carbonic acid dimethyl ester;
at 20 - 40 ℃;
for 1h;
Temperature;
Reagent/catalyst;
|
94.3% |
|
With
N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate;
In
N,N-dimethyl-formamide;
at 65 ℃;
for 5h;
Solvent;
|
90.5% |
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 36 - 48 ℃;
for 4.5h;
Temperature;
Large scale;
|
87% |
|
With
tetramethyl ammoniumhydroxide; potassium carbonate; carbonic acid dimethyl ester;
|
82% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 50 ℃;
for 16h;
|
72.6% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 50 ℃;
for 16h;
|
71% |
|
With
dmap; potassium carbonate;
In
1-methyl-pyrrolidin-2-one;
at 20 - 25 ℃;
for 5h;
Solvent;
|
266.3 g |
|
With
N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate;
In
N,N-dimethyl-formamide; toluene;
at 65 ℃;
|
-
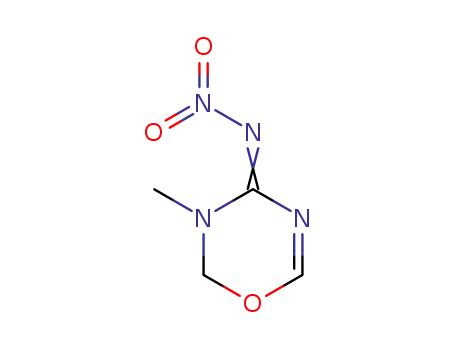
-
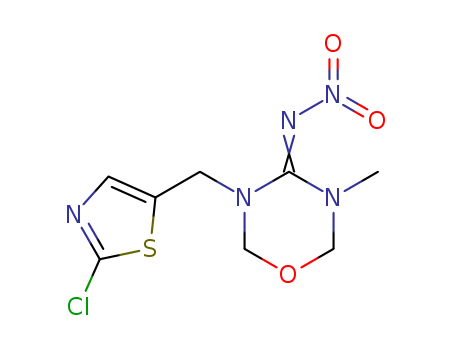
3-methyl-4-nitroimino-1,3,5-oxadiazine

-

-
105827-91-6
α-Cl-β-thiazolyl chloride

-

-
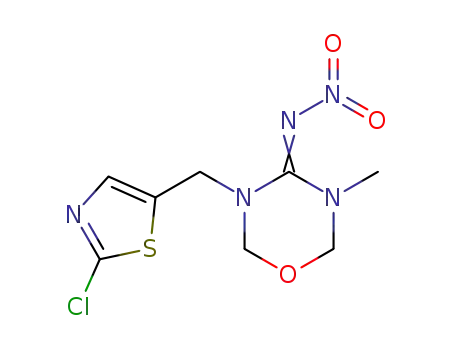
153719-23-4
thiamethoxam
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 30 - 70 ℃;
|
92.88% |
|
3-methyl-4-nitroimino-1,3,5-oxadiazine;
With
tetrabutylammomium bromide; potassium carbonate;
In
water; acetonitrile;
at 70 ℃;
for 1h;
α-Cl-β-thiazolyl chloride;
In
water; acetonitrile;
at 70 ℃;
for 5h;
|
153719-23-4 Upstream products
-
105827-91-6

α-Cl-β-thiazolyl chloride
-
153719-38-1

3,6-dihydro-3-methyl-N-nitro-2H-1,3,5-oxadiazin-4-amine
-
74-88-4
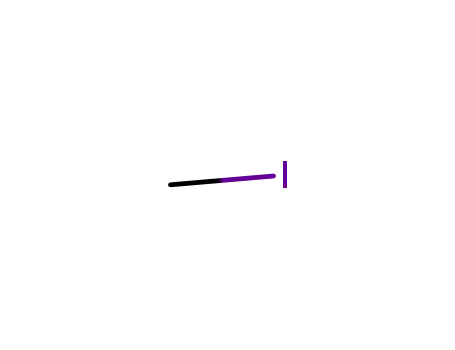
methyl iodide
-
135018-15-4
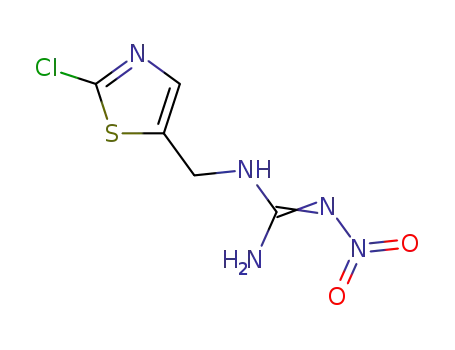
N-(2-chlorothiazol-5-ylmethyl)-N'-nitroguanidine
Relevant Products
-
Benzathine Cloxacillin
CAS:23736-58-5
-
dinotefuran
CAS:165252-70-0
-
Glycine ethyl ester hydrochloride
CAS:623-33-6