165252-70-0
- Product Name:dinotefuran
- Molecular Formula:C7H14N4O3
- Purity:99%
- Molecular Weight:202.213
Product Details;
CasNo: 165252-70-0
Molecular Formula: C7H14N4O3
Appearance: white crystalline powder
Buy Quality Quality Factory Supply dinotefuran 165252-70-0 with Cheap Price
- Molecular Formula:C7H14N4O3
- Molecular Weight:202.213
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:107.5oC
- Refractive Index:1.596
- Boiling Point:334.5 °C at 760 mmHg
- PKA:3.24±0.50(Predicted)
- Flash Point:156.1 °C
- PSA:91.47000
- Density:1.42 g/cm3
- LogP:0.68460
Dinotefuran(Cas 165252-70-0) Usage
|
Description |
Dinotefuran is a broad-spectrum insecticide, which is proposed for food uses in/on leafy vegetables (except Brassica) (group 4), and for use in professional turf management, professional ornamental production, and in the residential indoor, pet, lawn and garden markets. Dinotefuran is a neonicotinoid in the nitroguanidine sub-class, same as another insecticide clothianidin. |
|
Chemical Properties |
Technical dinotefuran is an odorless white crystalline solid. It has a solubility of 39.83 g/L in water, and is highly soluble in dichloromethane, acetone, methanol, and ethyl acetate. Technical dinotefuran has a melting point of 107.5°C, and a log Pow of -0.549 at 25°C. It is nonflammable, is not explosive to thermal, shock and frictional tests, and has a vapor pressure of <1.7 x 10-6 Pa at 25°C. |
|
Uses |
Dinotefuran is a contact insecticide without systemic effect that belongs to the chemical class of the neonicotinoids, which are among the most widely used insecticides in the world. It is highly effective against fleas but not against ticks or mites, or any internal parasites.It is often used in combination with a tickicide/acaricide (e.g. permethrin) or with an insect growth regulator (e.g. pyriproxyfen). So far it is not used in livestock or horses. |
|
Flammability and Explosibility |
Notclassified |
|
Agricultural Uses |
Dinotefuran is an insecticide: Controls insect pests such as aphids, whiteflies, thrips, leafhopper, leafminer, sawfly, mole cricket, white grubs, lacebugs, billbugs, beetles, mealybugs, sawfly larvae, and cockroaches in leafy vegetables, in residential and commercial buildings, outdoor uses for professional turf management, turf farms, professional ornamental production, golf courses, residential indoor, lawn and garden use [83] . Not listed for use in EU countries. Actively registered in the U.S. and other countries. |
|
Trade name |
ALBARIN?; MIKEBLOCK MTI-446?; SAFARI?; SPARKLE?; VENOM? |
|
Pharmacokinetics |
After topical administration to dogs and cats dinotefuran remains mostly on the animal's surface and is not absorbed. However a certain amount can be ingested through licking and grooming. In laboratory animals ingested dinotefuran was almost completely absorbed (>90%) quickly distributed throughout the whole body and also rapidly eliminated, mostly through urine in the form of unchanged dinotefuran. |
|
Carcinogenicity |
Dinotefuran has been classified as —Not likely to be carcinogenic to humans.“ This classification is based on the lack of evidence for carcinogenicity in mice and rats. |
|
Environmental Fate |
Dinotefuran is nontoxic to birds, mammals, fish and algae, but it is highly toxic to marine/estuarine mysid shrimp.Dinotefuran is highly toxic to honey bees by all routes of administration.The mean aerobic biodegradation half-life of dinotefuran measured in 2 soils was 81.5 days. If released into water, dinotefuran is not expected to adsorb to suspended solids and sediment.Dinotefuran was stable to hydrolysis over the pH range of 4 to 9, but was rapidly photolyzed in aqueous solution with a half-life of 1.8 days.Potential for bioconcentration in aquatic organisms is low.Dinotefuran may potentially be present in drinking water, given its high water solubility, high mobility in soils.Dinotefuran does not cumulate in the environment or in the food chain.Correct use of dinotefuran in dogs and cats is unlikely to result in any significant environmental pollution. |
|
Toxicity evaluation |
LD50 acute, rats, oral 2450 mg/kgD50 acute, rabbits, dermal >2000 mg/kgAs a general rule, dogs and cats tolerate dinotefuran quite well.Dinotefuran is slightly irritant to the eyes and the skin.Dinotefuran is not carcinogenic, teratogenic or mutagenic.Seven repeated dermal administrations of dinotefuran to kittens at 1X, 3X and 5X the recommended dose were well tolerated by the animals. Dry skin as well as soft, loose or liquid feces were reported in some treated kittens bur were of transient character. |
InChI:InChI=1/C7H14N4O3/c1-8-7(10-11(12)13)9-5-6-3-2-4-14-6/h6H,2-5H2,1H3,(H2,8,9,10)
165252-70-0 Relevant articles
Preparation method of dinotefuran
-
Paragraph 0013; 0054-0062; 0069-0074, (2021/08/07)
The invention provides a preparation met...
165252-70-0 Process route
-
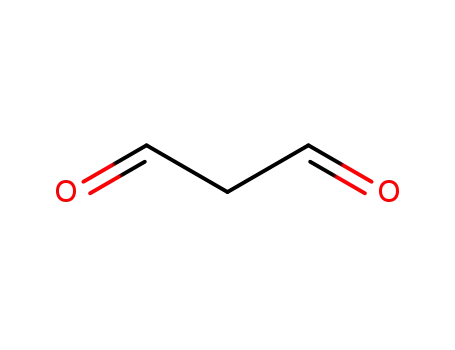
-
542-78-9
Malondialdehyde

-
-
C2H7N5O2

-
-
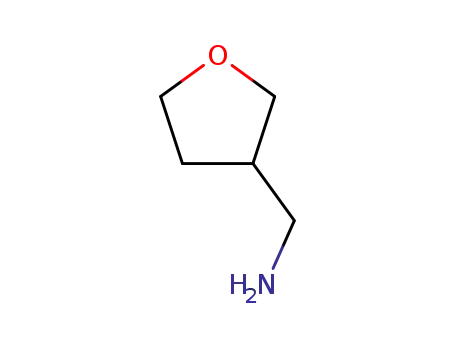
165253-31-6,1048962-84-0
(tetrahydro-3-furanyl)methylamine

-
-
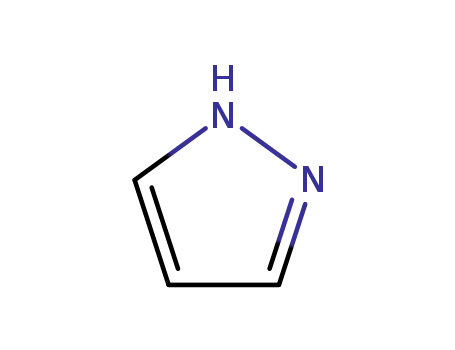
288-13-1
NH-pyrazole

-
-
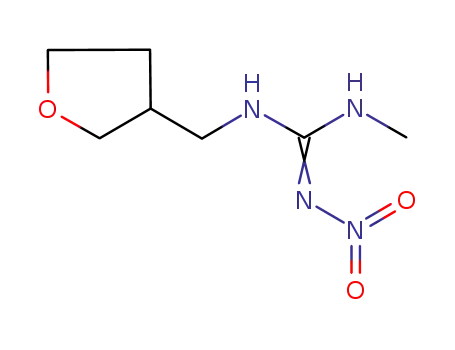
165252-70-0
dinetofuran
| Conditions | Yield |
|---|---|
|
Malondialdehyde; C2H7N5O2;
With
formic acid;
In
toluene;
at 60 ℃;
for 1h;
Molecular sieve;
(tetrahydro-3-furanyl)methylamine;
In
toluene;
at 60 ℃;
for 2h;
Molecular sieve;
|
83.1% |
-
-
C2H7N5O2

-
-
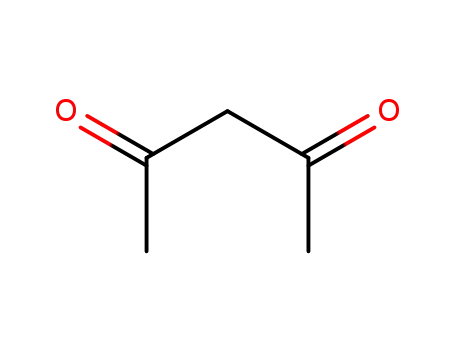
123-54-6,81235-32-7
acetylacetone

-

-
165253-31-6,1048962-84-0
(tetrahydro-3-furanyl)methylamine

-
-
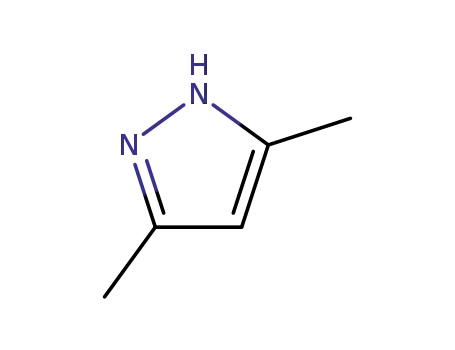
67-51-6
3,5-dimethyl-1H-pyrazole

-

-
165252-70-0
dinetofuran
| Conditions | Yield |
|---|---|
|
C2H7N5O2; acetylacetone;
With
formic acid;
In
toluene;
at 100 ℃;
for 1h;
Molecular sieve;
(tetrahydro-3-furanyl)methylamine;
In
toluene;
at 100 ℃;
for 2h;
Molecular sieve;
|
96% |
165252-70-0 Upstream products
-
123-54-6

acetylacetone
-
165253-31-6

(tetrahydro-3-furanyl)methylamine
-
1522-22-1
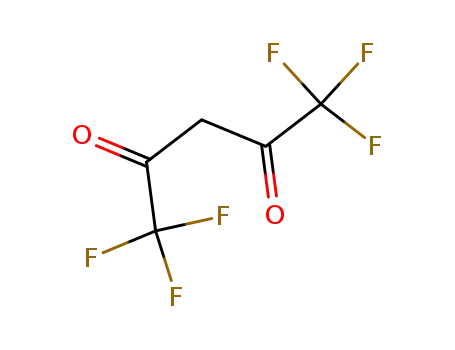
1,1,1,5,5,5-hexafluoroacetylacetone
-
542-78-9

Malondialdehyde
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
Norepinephrine Bitartrate
CAS:51-40-1
-
Thiamethoxam
CAS:153719-23-4