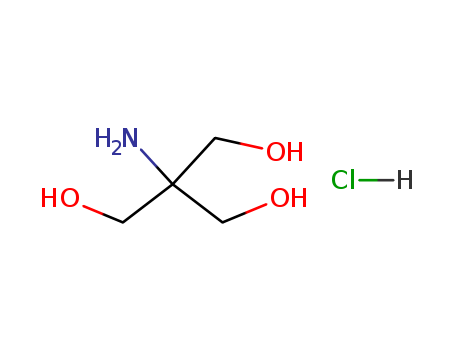
1185-53-1
- Product Name:TRIS hydrochloride
- Molecular Formula:C4H12ClNO3
- Purity:99%
- Molecular Weight:157.597
Product Details;
CasNo: 1185-53-1
Molecular Formula: C4H12ClNO3
Appearance: White crystalline powder
Factory Sells Trustworthy Factory Supply TRIS hydrochloride 1185-53-1 with Safe Shipping
- Molecular Formula:C4H12ClNO3
- Molecular Weight:157.597
- Appearance/Colour:White crystalline powder
- Vapor Pressure:0Pa at 20℃
- Melting Point:150-152 ºC
- Boiling Point:357 ºC at 760 mmHg
- PKA:8.1(at 25℃)
- Flash Point:169.7 ºC
- PSA:86.71000
- Density:1.05g/mLat 20°C
- LogP:-0.83690
2-Amino-2-(hydroxymethyl)-1,3-propanediol hydrochloride(Cas 1185-53-1) Usage
|
Chemical Properties |
White crystalline powder. Tris HCl has a pKa of 8.08 at 25°C and pH range of 7.0 to 9.0 that coincides with the physiological pH of most living organisms because of which it is commonly used as a component of buffer solutions in biology, biochemistry and molecular biology applications. Tris salts are also used for crystallization of proteins at various pH values. Neither Tris base nor Tris hydrochloride by itself provide adequate buffering capacity. Generally the two need to be mixed together to provide a buffer with pH between 7 and 9 to provide adequate buffering. |
|
Uses |
TRIS hydrochloride is a high quality buffer component used in SDS-PAGE. For precise applications, use a carefully calibrated pH meter with a glass/calomel combination electrode. The pH values of all buffers are temperature and concentration dependent. For Tris buffers, pH increases about 0.03 unit per °C decrease in temperature, and decreases 0.03-0.05 unit per ten-fold dilution. It is also used in the robust generation of transgenic mice. |
|
Application |
TRIS hydrochloride is highly soluble in water and is useful in the pH range 7.0-9.0. It is used in the preparation of Laemmli buffer, one of the most common SDS-PAGE buffers. In addition, Tris can also be used for many custom running and loading buffers, most often with glycine and SDS. The use of single-junction pH electrodes that contain silver should be avoided when determining the pH of a solution containing Tris buffer. |
|
Preparation |
The preparation of TRIS hydrochloride is as follows:After reacting 2-amino-1,3-propanediol (2.50 g, 27.44 mmol) and formic acid (1.26 g, 27.44 mmol) in 50 ml of water at room temperature for 3 hours, water was distilled off under reduced pressure, A liquid was obtained. By washing the obtained liquid,An ammonium formate salt (3.76 g, 27.44 mmol) was obtained as a colorless transparent liquid. |
|
General Description |
Trizma? hydrochloride is mainly required for preparation of buffer at physiological range of 7.3 to 7.5. The prepared buffer is compatible with biological fluids. It is of importance to laboratories as a standard pH solution. |
|
Purification Methods |
Crystallise the salt from 50% EtOH, then from 70% EtOH. TRIS-hydrochloride is also available commercially in a highly pure state. Otherwise, recrystallise it from 50% EtOH, then 70% EtOH, and dry it below 40o to avoid risk of decomposition. [Beilstein 4 H 304.] |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C4H11NO3.ClH/c5-4(1-6,2-7)3-8;/h6-8H,1-3,5H2;1H/p-1
1185-53-1 Relevant articles
The hydrophilic ionic liquid at room temperature and its use (by machine translation)
-
Paragraph 0099; 0100; 0237; 0254, (2018/10/24)
PROBLEM TO BE SOLVED: To provide a novel...
PRIMER FOR AMPLIFICATION OF RRNA OR BACTERIUM BELONGING TO THE GENUS LEGIONELLA, DETECTION METHOD, AND DETECTION KIT
-
, (2010/12/29)
To detect a bacterium belonging to the g...
Phenoxybenzylamine derivatives as SSRls
-
, (2008/06/13)
A compound of general formula (I) wherei...
Stereoelectronic control of oxazolidine ring-opening: Structural and chemical evidences
Sélambarom, Jimmy,Monge, Sophie,Carré, Francis,Roque, Jean Pierre,Pavia, André A
, p. 9559 - 9566 (2007/10/03)
Ring opening of oxazolidines derived fro...
1185-53-1 Process route
-
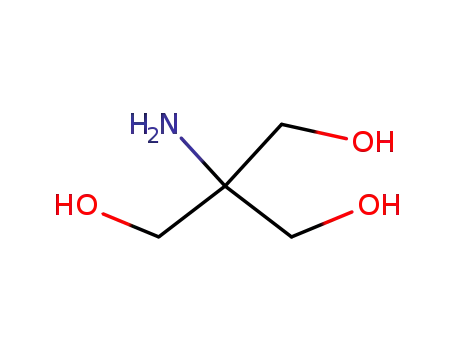
-
77-86-1
2-amino-2-hydroxymethyl-1,3-propanediol

-
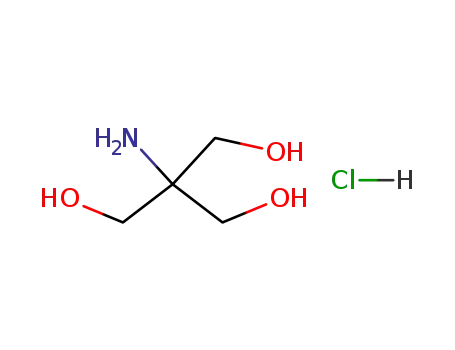
-
1185-53-1
tris hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
at 20 ℃;
for 3h;
|
-
![cis-2,8-diphenyl-5-hydroxymethyl-1-aza-3,7-dioxabicyclo[3.3.0]octane](/upload/2024/4/6eb5ec91-d5ee-48c4-8493-7f8830448ac0.png)
-
cis-2,8-diphenyl-5-hydroxymethyl-1-aza-3,7-dioxabicyclo[3.3.0]octane

-

-
1185-53-1
tris hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol;
for 1h;
Heating;
|
80% |
1185-53-1 Upstream products
-
77-86-1

2-amino-2-hydroxymethyl-1,3-propanediol
1185-53-1 Downstream products
-
100-15-2
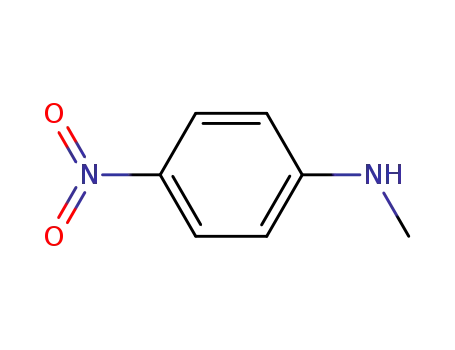
N-methyl(p-nitroaniline)
-
53104-32-8
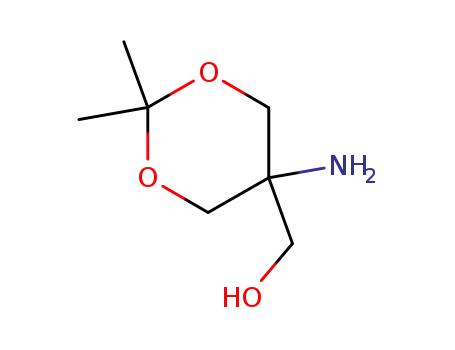
(5-amino-2,2-dimethyl-1,3-dioxan-5-yl)methanol
-
51430-74-1
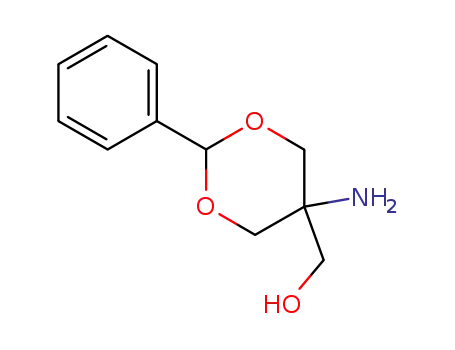
5-amino-5-hydroxymethyl-2-phenyl-1,3-dioxane
-
28384-96-5
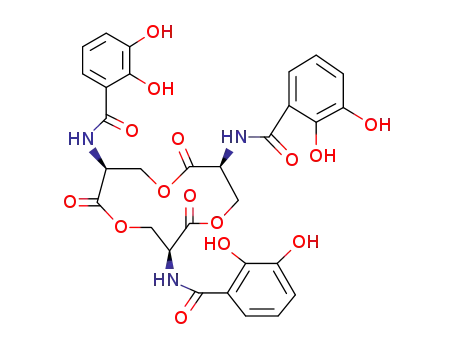
L-enterobactin
Relevant Products
-
Propranolol Hydrochloride
CAS:3506-09-0
-
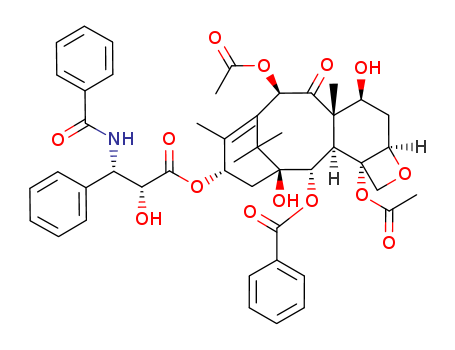
Paclitaxel
CAS:33069-62-4
-
Vitamin B4
CAS:73-24-5