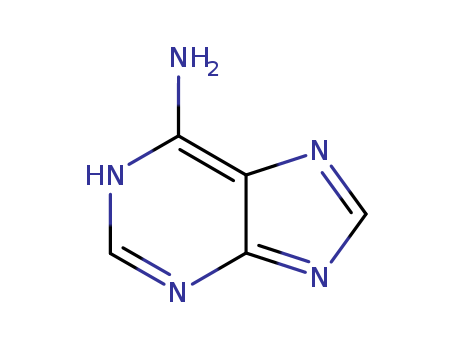
73-24-5
- Product Name:Vitamin B4
- Molecular Formula:C5H5N5
- Purity:99%
- Molecular Weight:135.128
Product Details;
CasNo: 73-24-5
Molecular Formula: C5H5N5
Appearance: white to almost white crystalline powder
Hot Sale Trustworthy Manufacturer Supply Vitamin B4 73-24-5 In Stock
- Molecular Formula:C5H5N5
- Molecular Weight:135.128
- Appearance/Colour:white to almost white crystalline powder
- Vapor Pressure:0.00172mmHg at 25°C
- Melting Point:>360 °C(lit.)
- Refractive Index:1.954
- Boiling Point:553.491 °C at 760 mmHg
- PKA:4.12(at 25℃)
- Flash Point:322.71 °C
- PSA:80.48000
- Density:1.612 g/cm3
- LogP:0.51630
Adenine(Cas 73-24-5) Usage
|
Chemical Description |
Adenine is a purine base found in DNA and RNA. |
|
DNA Composition and Nano-structuring |
Adenine (A) is one of the four nucleobases found in DNA, along with Thymine (T), Cytosine (C), and Guanine (G). DNA oligomers composed of these nucleobases possess precise nano-structuring capabilities. Adenine's role in DNA allows for facile sequence programmability and specific hybridization properties with complementary sequences. |
|
Chiral Assembly Structures |
Adenine's specific hybridization properties enable the design of chiral assembly structures using DNA. Studies have utilized adenine's interaction with complementary sequences to assemble nanoparticles into tetrahedral pyramidal shapes with distinct chirality. By designing attached oligomer sequences on different nanoparticles, chiral assembly structures with four distinct constituent nanoparticles have been constructed. |
|
Surface Interaction with Gold |
Adenine exhibits high affinity with gold surfaces, followed by Cytosine, Guanine, and Thymine. This nucleobase-dependent surface interaction allows for synthetic control of nanomaterials. The kinetics and dynamics of adenine's interaction with gold surfaces play a crucial role in inducing single-nanoparticle morphology control in both monometallic and bimetallic systems. |
|
Variations in Bacterial Viruses |
In bacterial viruses, variations in DNA bases such as Adenine help them escape degradation by bacterial restriction enzymes. For example, in the genome of cyanophage S-2L, Adenine is completely replaced by diaminopurine (Z), which forms non鈥揂 watson-Crick base pairing with Thymine. |
|
Applications in Organic Electronics |
Adenine, as a constituent of DNA and RNA polymers, exhibits favorable properties for applications in organic electronics. Adenine's low oxidation potential makes it suitable for redox reactions. Additionally, adenine has been used as an electron-blocking layer in organic light-emitting diodes (OLEDs), leading to performance enhancements in terms of current efficiency and external quantum efficiency. |
|
Description |
adenine is one of the purine nitrogenous bases that composes DNA and RNA; composed of two carbon–nitrogen rings. Adenine bonds with thymine in DNA and with uracil in RNA (see base pairing rule); it is also a major component of other molecules such as adenosine triphosphate. |
|
Chemical Properties |
Adenine is a prominent member of the family of naturally occurring purines. Adenine occurs not only in ribonucleic acids (RNA), and deoxyribonucleic acids (DNA), but in nucleosides, such as adenosine, and nucleotides, such as adenylic acid, which may be linked with enzymatic functions quite apart from nucleic acids. Adenine, in the form of its ribonucleotide, is produced in mammals and fowls endogenously from smaller molecules and no nutritional essentiality is ascribed to it. In the nucleosides, nucleotides, and nucleic acids, the attachment or the sugar moiety is at position 9.The purines and pyrimidines absorb ultraviolet light readily, with absorption peaks at characteristic frequencies. This has aided in their identification and quantitative determination. |
|
Physical properties |
Adenine is a white to almost white crystalline powder that is an important biological compound found in deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and adenosine triphosphate (ATP). It was once commonly referred to as vitamin B4 but is no longer considered a vitamin. Adenine is derived from purine. Purine is a heterocyclic compound. |
|
History |
Adenine is one of the two purines found in DNA and RNA. The other is guanine. Adenine and guanine are called bases in reference to DNA and RNA. A nucleic acid base attached to ribose forms a ribonucleoside. Adenine combined with ribose produces the nucleoside adenosine. |
|
Uses |
Adenine is used as an active component of boron-deficient media to grow yeast in order to assess whether yeast growth is stimulated by boron. It is useful as a local antiseptic and vitamin B4. Further, it is used in the microbial determination of niacin. It is also employed as a food supplement for adult rats to investigate the effects of dietary adenine overload. In addition to this, it is used in the production of nucleotides of the nucleic acids. |
|
Definition |
ChEBI: Adenine is the parent compound of the 6-aminopurines, composed of a purine having an amino group at C-6. It has a role as a human metabolite, a Daphnia magna metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a purine nucleobase and a member of 6-aminopurines. It derives from a hydride of a 9H-purine. |
|
Application |
Widespread throughout animal and plant tissues combined with niacinamide, d-ribose, and phosphoric acids; a constituent of nucleic acids and coenzymes, such as codehydrase I and II, adenylic acid, coa laninedehydrase. It is used in microbial determination of niacin; in research on heredity, virus diseases, and cancer. |
|
General Description |
Adenine is a purine nucleobase. It is part of DNA, and RNA. Adenine is also a component of cofactors (NAD, FAD) and signaling molecules (cAMP). It is a nitrogenous base found in DNA and RNA. It is also a constituent of certain coenzymes and when combined with the sugar ribose it forms the nucleoside adenosine found in AMP, ADP, and ATP. Adenine has a purine ring structure. It is one of the major component bases ofnucleotides and the nucleic acidsDNA and RNA. |
|
Biochem/physiol Actions |
Adenine is essential for many in vivo and in vitro biochemical processes. Adenine is converted to adenosine with ribose. On phosphorylation, it forms AMP, ADP and ATP. ATP is the energy currency of the cell and is required during cellular metabolism. Adenine is metabolized to is 2,8-dihydroxyadenine, which on accumulation in proximal tubules leads to the induction of chronic kidney disease (CKD) with severe anemia in rats. Adenine based derivatives elicit antiviral functionality against dsDNA viruses and are exploited for generating antiviral scaffolds. |
|
Safety Profile |
Poison by intraperitoneal route. Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx,. |
|
Purification Methods |
Crystallise adenine from distilled water. [Beilstein 26 III/IV 3561.] |
InChI:InChI=1/C5H5N5/c6-4-3-5(9-1-7-3)10-2-8-4/h1-3H,(H2,6,7,8,9,10)
73-24-5 Relevant articles
Using conformationally locked nucleosides to calibrate the anomeric effect: Implications for glycosyl bond stability
Moon, Hyung Ryong,Siddiqui, Maqbool A.,Sun, Guangyu,Filippov, Igor V.,Landsman, Nicholas A.,Lee, Yi-Chien,Adams, Kristie M.,Barchi Jr., Joseph J.,Deschamps, Jeffrey R.,Nicklaus, Marc C.,Kelley, James A.,Marquez, Victor E.
, p. 6707 - 6717 (2010)
Steric and electronic parameters, such a...
Detecting ricin: Sensitive luminescent assay for ricin A-chain ribosome depurination kinetics
Sturm, Matthew B.,Schramm, Vern L.
, p. 2847 - 2853 (2009)
Ricin is a family member of the lethal r...
Sources of 2,5-diaminoimidazolone lesions in DNA damage initiated by hydroxyl radical attack
Thomas, Caroline Suzanne,Pollard, Hannah Catherine,Razskazovskiy, Yuriy,Roginskaya, Marina
, p. 517 - 524 (2020/09/07)
The present study reports radiation-chem...
Plasmonic Hot Electron-Mediated Hydrodehalogenation Kinetics on Nanostructured Ag Electrodes
Liu, Jia,Cai, Zhuan-Yun,Sun, Wei-Xin,Wang, Jia-Zheng,Shen, Xiao-Ru,Zhan, Chao,Devasenathipathy, Rajkumar,Zhou, Jian-Zhang,Wu, De-Yin,Mao, Bing-Wei,Tian, Zhong-Qun
supporting information, p. 17489 - 17498 (2020/11/12)
An attractive field of plasmon-mediated ...
Adenine intermediate pyrimidine-azo compound and preparation method thereof
-
Paragraph 0023, (2017/07/14)
The invention relates to an adenine inte...
Total chemical synthesis method of adenine
-
Paragraph 0017; 0020; 0024, (2017/08/29)
The invention relates to a total chemica...
73-24-5 Process route
-
-
7664-93-9
sulfuric acid

-
-
7732-18-5
water

-
-
2457-80-9
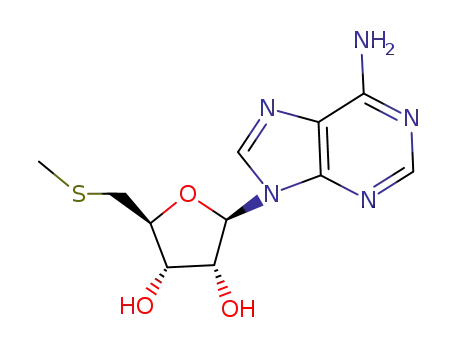
5'-Deoxy-5'-methylthioadenosine

-
-
23656-67-9,23656-91-9
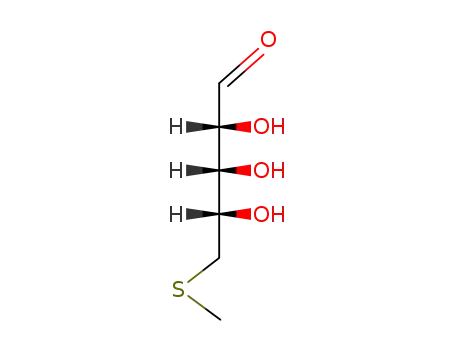
S-methyl-5-thio-D-ribose

-
-
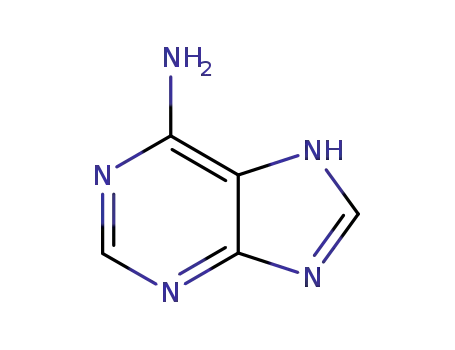
73-24-5,71660-29-2,71660-30-5
7H-purin-6-ylamine
| Conditions | Yield |
|---|---|
|
at 100 ℃;
Hydrolysis;
|
-
-
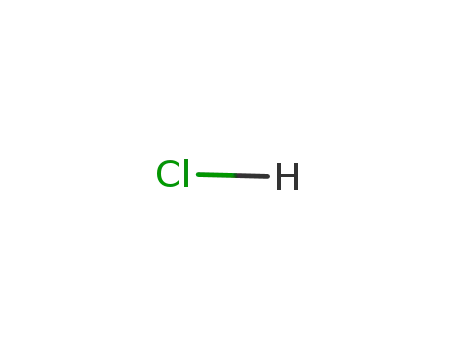
7647-01-0,15364-23-5
hydrogenchloride

-

-
7732-18-5
water

-

-
2457-80-9
5'-Deoxy-5'-methylthioadenosine

-

-
23656-67-9,23656-91-9
S-methyl-5-thio-D-ribose

-

-
73-24-5,71660-29-2,71660-30-5
7H-purin-6-ylamine
| Conditions | Yield |
|---|---|
|
|
73-24-5 Upstream products
-
1198-83-0
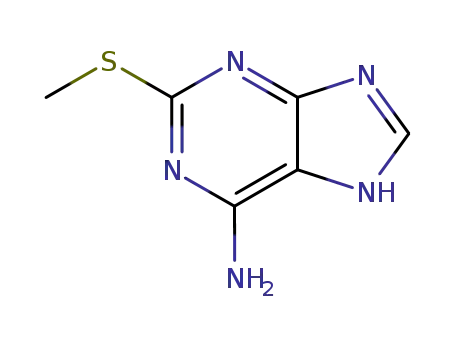
2-(methylthio)-7H-purin-6-amine
-
5122-36-1
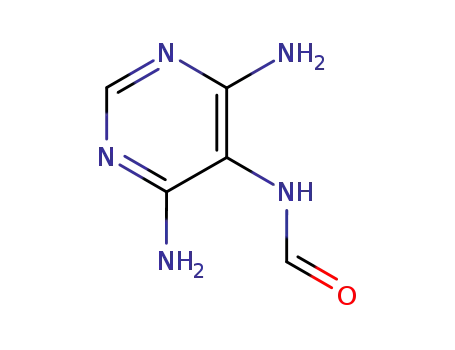
N-(4,6-diaminopyrimidin-5-yl)formamide
-
409110-30-1
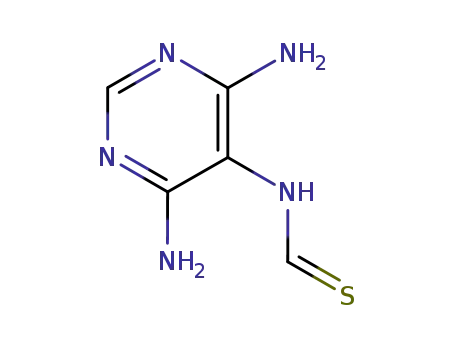
N-(4,6-diamino-pyrimidin-5-yl)-thioformamide
-
3647-48-1
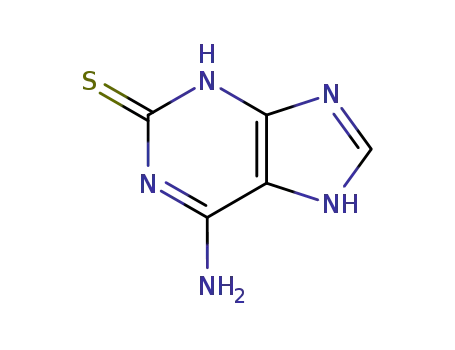
6-amino-3,7-dihydro-purine-2-thione
73-24-5 Downstream products
-
525-79-1
kinetin
-
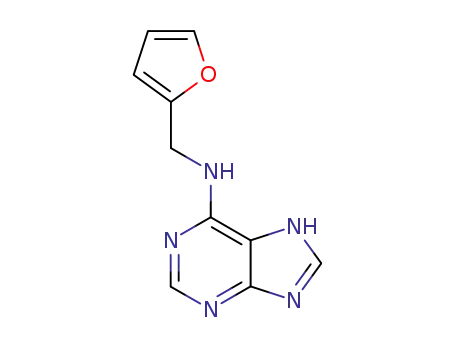
65316-39-4
N-(7(9)H-purin-6-yl)-furan-2-carboxamide
-
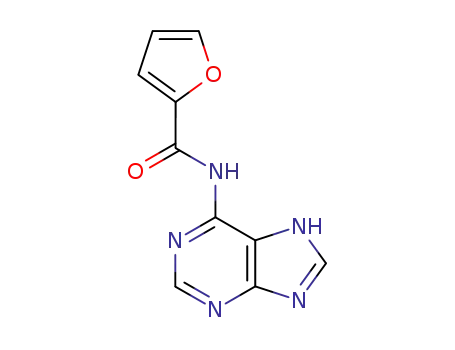
65316-41-8
N-(7(9)H-purin-6-yl)-thiophene-2-carboxamide
-
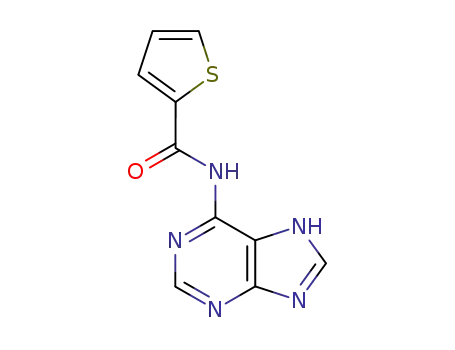
5458-50-4
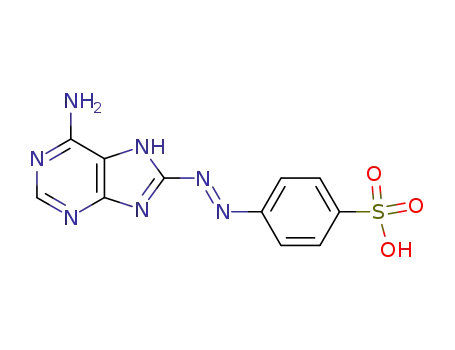
4-(6-amino-7H-purin-8-ylazo)-benzenesulfonic acid
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
TRIS hydrochloride
CAS:1185-53-1
-
1H-Indazole-3-carboxamide
CAS:121061-98-1