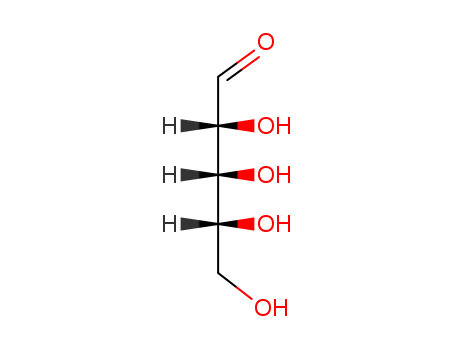
Product Details;
CasNo: 50-69-1
Molecular Formula: C5H10O5
Appearance: white powder
Hot Sale Quality Manufacturer Supply D-ribose 50-69-1 with Competitive Price
- Molecular Formula:C5H10O5
- Molecular Weight:150.131
- Appearance/Colour:white powder
- Vapor Pressure:3.6E-07mmHg at 25°C
- Melting Point:95 °C
- Refractive Index:-21 ° (C=1, H2O)
- Boiling Point:375.4 °C at 760 mmHg
- PKA:12.46±0.20(Predicted)
- Flash Point:180.8 °C
- PSA:97.99000
- Density:1.681 g/cm3
- LogP:-2.73970
D-Ribose(Cas 50-69-1) Usage
|
Identification and Nomenclature |
It is identified as a D-ribose and an aldehydo-ribose, being the enantiomer of an aldehydo-L-ribose. Its systematic name is (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal. |
|
Occurrence |
Found naturally in organisms such as Streptomyces sporangiiformans and Arabidopsis thaliana. |
|
Versatility |
Known by various names including ribose, aldehydo-d-ribo-pentose, and ribo-2,3,4,5-tetrahydroxyvaleraldehyde. Its unique structure and properties make it useful in pharmaceuticals, biochemistry, and organic synthesis. |
|
Precursor to D-Ribose |
Serves as a precursor to D-ribose, which is a crucial component of ribonucleic acid (RNA) and certain coenzymes. |
|
Cyclic Forms |
Like most sugars, ribose exists as a mixture of cyclic forms in equilibrium with its linear form, and these readily interconvert especially in aqueous solution. In its linear form, ribose can be recognised as the pentose sugar with all of its hydroxyl functional groups on the same side in its Fischer projection. d-Ribose has these hydroxyl groups on the right hand side and is associated with the systematic name (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal, whilst l-ribose has its hydroxyl groups appear on the left hand side in a Fischer projection. |
|
Description |
D-ribose is an important five-carbon monosaccharide with the chemical formula C5H10O5. It is an important constituent of ribonucleic acid (RNA) and ATP, and plays an important role in the formation of life. It is also an important pharmaceutical intermediate for the production of various nucleic acid drugs. |
|
Chemical Properties |
D-Ribose is a five-carbon sugar with strong water solubility and sweet taste, also known as D-ribofuranose. It is used by all living cells and is an essential component in living organisms for energy production. It is widely present in the furan form. |
|
Uses |
D-Ribose is produced by microorganism fermentation of glucose in a fermentation culture medium without adding calcium carbonate. |
|
Preparation |
D-Ribose is obtained by using D-glucose as a raw material, inserting bacteria or Bacillus subtilis for fermentation, and separating and refining the fermentation product.(By fermentation technology) |
|
Definition |
ChEBI: D-ribose is a ribofuranose having D-configuration. It is a naturally occurring monosaccharide found in the cells and particularly in the mitochondria is essential in energy production. |
|
Biological Activity |
D-ribose is a natural sugar that our bodies produce for a variety of purposes most notably encrgy in the form of ATP(adenosine triphosphate), which powers every cell in our body. Ribose is crucial for the synthesis of energy (ATP) and without it, your cells run out of energy and the health of the cell is compromised. Supplementing with ribose improves cell function by restoring energy. One of the most vital organs to suffer energy depletion is the heart. That is why so much of the research on D-ribose has focused on the impact of supplement ribose in heart disease. Although ribose is classified as a sugar, when taken orally, it does not have any impact on blood sugar like glucose. Instead, it is used for energy production and energy recovery. Every cell in the body has the capacity to make ribose. But more ribose may be needed than the cell can produce when cells are metabolically stressed, such as with strenuous exercise or disease. When the cells are metabolically stressed they tend to consume more glucose rather than producing more ribose. This can dramatically affect recovery. |
|
Biochem/physiol Actions |
Ribose is an aldopentose monosaccharide that is phosphorylated into D-ribose 5-phosphate by ribokinase. |
|
Side effects |
D-ribose is a dietary supplement approved by the US FDA. It is generally considered safe for dosage of ribose is less than 15g/d. However, possible side effects include diarrhea, stomach discomfort, nausea, headache, and low blood sugar. |
|
Purification Methods |
Crystallise -D(-)-ribose from aqueous 80% EtOH, dry it under vacuum at 60o over P2O5 and store it in a vacuum desiccator. It exhibits a complex mutarotation with : [] D 10 -23.1o (1.5minutes), -21.3o (5minutes), -19.5o (10minutes), -19.1o (30minutes), -21.2o (60minutes), -23.1o (120minutes), -23.7o (300minutes), (c 4.5, H2O) [Phelps et al. J Am Chem Soc 56 748 1934]. 1H NMR in D2O at 44o shows 17% -pyranose, 59% -pyranose, 9% -furanose and 15% -furanose forms with furanose -H at 5.34ppm (J 3.0Hz) and -H at 5.31 (J 1.7Hz) [Angyal Adv Carbohydr Chem 42 15 1984, Angyal & Pickles Aust J Chem 25 1711 1972]. The phenylhydrazone crystallises from aqueous pyridine in yellow needles, m 163-164o, and the benzylphenylhydrazone has m 127-128o [Snowden J Am Chem Soc 72 808 1950.] [Beilstein 1 IV 4211.] |
InChI:InChI=1/C5H10O5/c6-1-2-3(7)4(8)5(9)10-2/h2-9H,1H2/t2-,3-,4-,5-/m1/s1
50-69-1 Relevant articles
Thermal Behavior of d-Ribose Adsorbed on Silica: Effect of Inorganic Salt Coadsorption and Significance for Prebiotic Chemistry
Akouche, Mariame,Jaber, Maguy,Zins, Emilie-Laure,Maurel, Marie-Christine,Lambert, Jean-Francois,Georgelin, Thomas
, p. 15834 - 15846 (2016)
Understanding ribose reactivity is a cru...
Comprehensive investigation of the energetics of pyrimidine nucleoside formation in a model prebiotic reaction
Sheng, Yinghong,Bean, Heather D.,Mamajanov, Irena,Hud, Nicholas V.,Leszczynski, Jerzy
, p. 16088 - 16095 (2009)
The problem of β-nucleoside formation un...
New bisamide compounds from the bark of Aglaia eximia (Meliaceae)
Sianturi, Julinton,Purnamasari, Mayshah,Darwati,Harneti, Desi,Mayanti, Tri,Supratman, Unang,Awang, Khalijah,Hayashi, Hideo
, p. 297 - 301 (2015)
Two new bisamide compounds, eximiamide A...
Biosynthesis of anti-HCV compounds using thermophilic microorganisms
Rivero, Cintia W.,De Benedetti, Eliana C.,Sambeth, Jorge E.,Lozano, Mario E.,Trelles, Jorge A.
, p. 6059 - 6062 (2012)
This work describes the application of t...
Isochromans and related constituents from the endophytic fungus Annulohypoxylon truncatum of Zizania caduciflora and their anti- inflammatory effects
Li, Wei,Lee, Changyeol,Bang, Sung Hee,Ma, Jin Yeul,Kim, Soonok,Koh, Young-Sang,Shim, Sang Hee
, p. 205 - 209 (2017)
Six new isochroman derivatives (annulohy...
-
Sato
, (1951)
-
Studies on the constant-current electrolytic reduction of D-ribonolactone to D-ribose. II. Application of amine salts as electrolyte
Matsumoto,Miyazaki
, p. 991 - 993 (1967)
-
-
Steiger
, p. 189,191 (1936)
-
Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JJM22
Wu, Jia-Ting,Zheng, Cai-Juan,Zhang, Bin,Zhou, Xue-Ming,Zhou, Qi,Chen, Guang-Ying,Zeng, Zhuo-Er,Xie, Jin-Long,Han, Chang-Ri,Lyu, Ji-Xing
, p. 34 - 40 (2019)
Two new compounds (1 and 2), together wi...
Biochemical characterization of a recombinant acid phosphatase from Acinetobacter baumannii
Smiley-Moreno, Elizabeth,Smith, Douglas,Yu, Jieh-Juen,Cao, Phuong,Arulanandam, Bernard P.,Chambers, James P.
, (2021/06/09)
Genomic sequence analysis of Acinetobact...
Kinase-Inhibitory Nucleoside Derivatives from the Pacific Bryozoan Nelliella nelliiformis
Bracegirdle, Joe,Gordon, Dennis P.,Harvey, Joanne E.,Keyzers, Robert A.
, p. 547 - 551 (2020/03/19)
Marine organisms are a valuable source o...
Hypoglycemic triterpenoid glycosides from Cyclocarya paliurus (Sweet Tea Tree)
Chen, Zuhui,Li, Jing,Lv, Wenyan,Sun, Huihui,Tan, Jie,Wang, Wenxuan,Wu, Jianping,Xu, Jialing,Xu, Kangping,Xuan, Tongyao,Yang, Zhichun,Ye, Zijun,Zhu, Hui,Zou, Zhenxing
, (2020/01/06)
Four new rarely occurred seco-dammarane ...
ELECTROCHEMICAL METHODS AND SYSTEMS FOR PRODUCING MONOSACCHARIDES
-
Paragraph 0067-0068, (2020/07/15)
The present disclosure is related to ele...
50-69-1 Process route
-
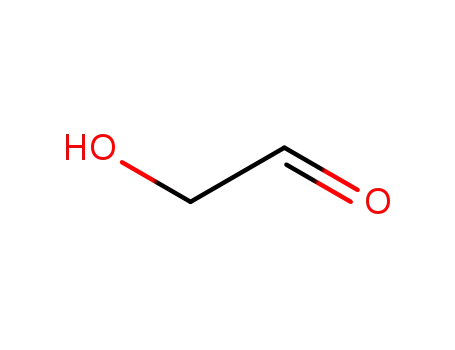
-
141-46-8
Glycolaldehyde

-
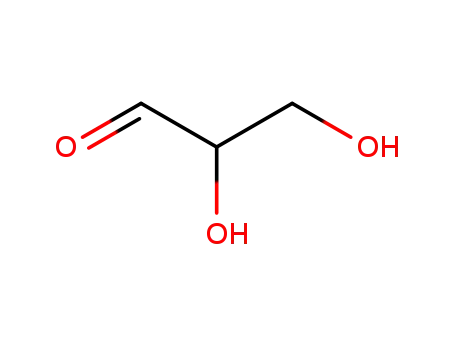
-
56-82-6,367-47-5,26793-98-6
Glyceraldehyde

-
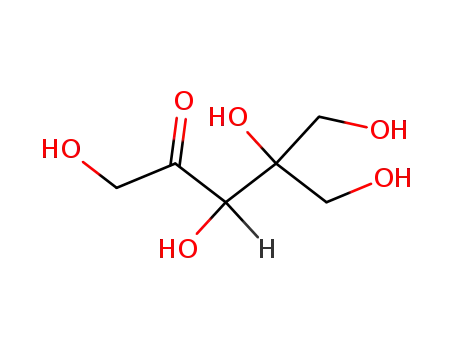
-
470-14-4
DL-dendroketose

-

-
20235-19-2
arabinose

-
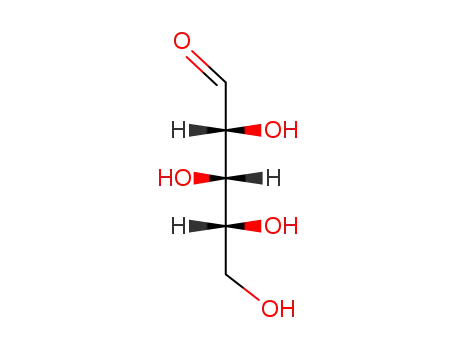
-
41247-05-6
(+/-)-xylose

-
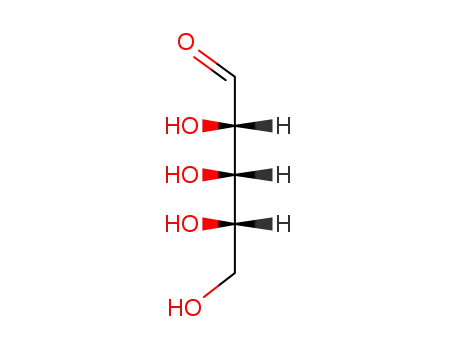
-
50-69-1,58-86-6,65-42-9,147-81-9,609-06-3,1114-34-7,1949-78-6,5328-37-0,10323-20-3,20235-19-2,24259-59-4,25990-60-7,34466-20-1,41247-05-6,53106-52-8,55058-43-0,80952-57-4
rac-Ribose

-
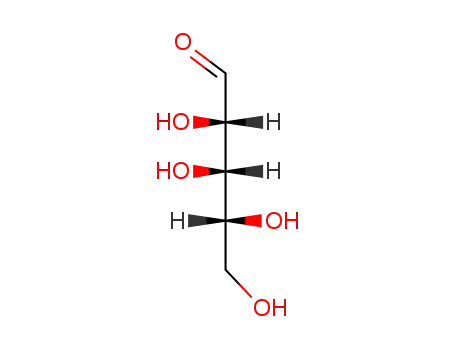
-
50-69-1,58-86-6,65-42-9,147-81-9,609-06-3,1114-34-7,1949-78-6,5328-37-0,10323-20-3,20235-19-2,24259-59-4,25990-60-7,34466-20-1,41247-05-6,53106-52-8,55058-43-0,80952-57-4
rac-lyxose

-
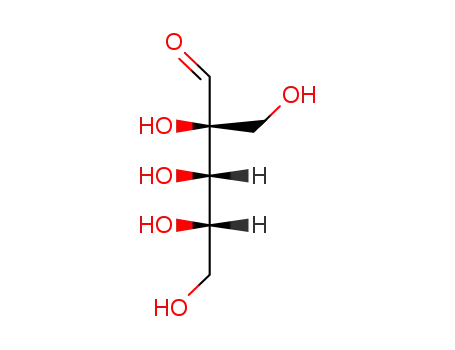
-
4072-92-8,4096-61-1,4211-40-9,4573-78-8,5612-45-3,6747-98-4,90409-92-0
Dendroaldose
| Conditions | Yield |
|---|---|
|
With
calcium hydroxide;
at 23 ℃;
Mechanism;
Kinetics;
|
-

-
141-46-8
Glycolaldehyde

-

-
56-82-6,367-47-5,26793-98-6
Glyceraldehyde

-

-
470-14-4
DL-dendroketose

-

-
20235-19-2
arabinose

-

-
41247-05-6
(+/-)-xylose

-

-
50-69-1,58-86-6,65-42-9,147-81-9,609-06-3,1114-34-7,1949-78-6,5328-37-0,10323-20-3,20235-19-2,24259-59-4,25990-60-7,34466-20-1,41247-05-6,53106-52-8,55058-43-0,80952-57-4
rac-Ribose

-

-
50-69-1,58-86-6,65-42-9,147-81-9,609-06-3,1114-34-7,1949-78-6,5328-37-0,10323-20-3,20235-19-2,24259-59-4,25990-60-7,34466-20-1,41247-05-6,53106-52-8,55058-43-0,80952-57-4
rac-lyxose

-

-
4072-92-8,4096-61-1,4211-40-9,4573-78-8,5612-45-3,6747-98-4,90409-92-0
Dendroaldose
| Conditions | Yield |
|---|---|
|
With
calcium hydroxide;
at 23 ℃;
Mechanism;
Kinetics;
|
50-69-1 Upstream products
-
110-86-1
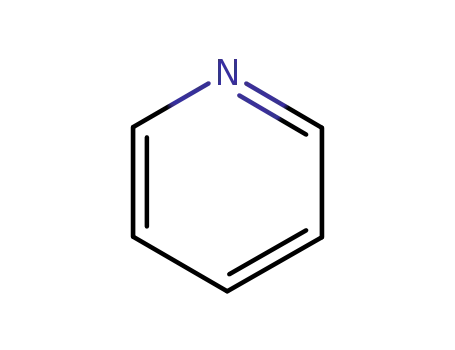
pyridine
-
488-84-6
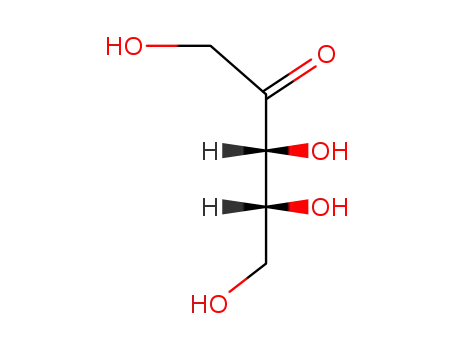
D-ribulose
-
551-84-8
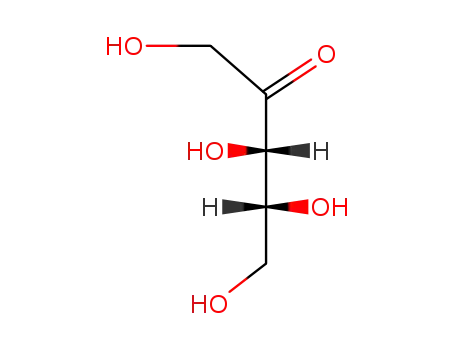
xylulose
-
75-52-5
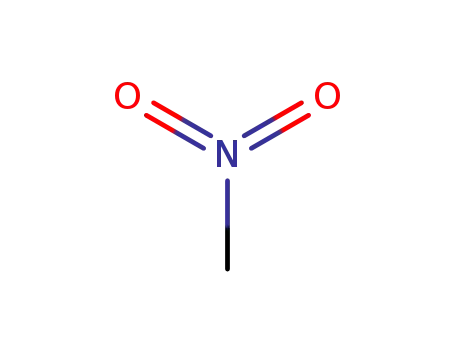
nitromethane
50-69-1 Downstream products
-
488-84-6

D-ribulose
-
13035-61-5
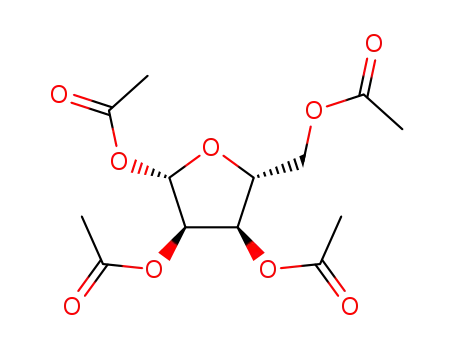
1,2,3,5-tetraacetylribose
-
50730-26-2
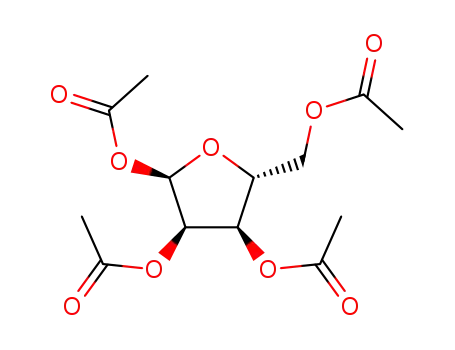
tetra-O-acetyl-D-ribofuranose
-
4257-95-8
1,2,3,4-tri-O-acetyl-α-D-ribopyranose
Relevant Products
-
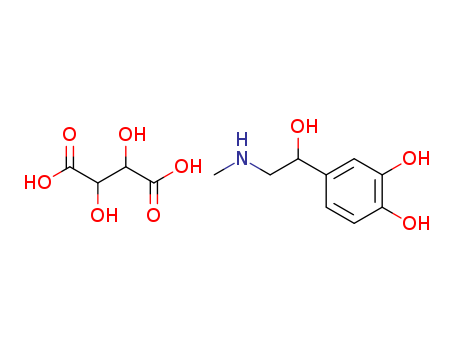
Epinephrine bitartrate
CAS:51-42-3
-
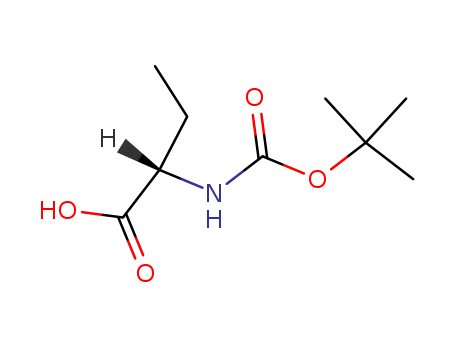
Boc-2-aminobutanoic acid
CAS:34306-42-8