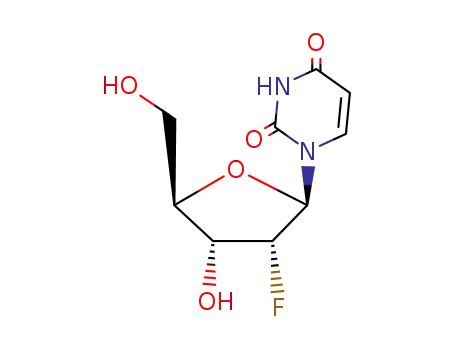
10212-20-1
- Product Name:4-Amino-1-[(2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one
- Molecular Formula:C9H12FN3O4
- Purity:99%
- Molecular Weight:245.21
Product Details;
CasNo: 10212-20-1
Molecular Formula: C9H12FN3O4
Appearance: White or almost white crystalline powder
Factory Sells Chinese Manufacturer Supply 4-Amino-1-[(2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one 10212-20-1 On Stock
- Molecular Formula:C9H12FN3O4
- Molecular Weight:245.21
- Appearance/Colour:White or almost white crystalline powder
- Melting Point:167 °C
- Refractive Index:80 ° (C=1, MeOH)
- Boiling Point:500.1 °C at 760 mmHg
- PKA:12.84±0.70(Predicted)
- Flash Point:256.2 °C
- PSA:110.60000
- Density:1.82 g/cm3
- LogP:-1.00460
4-Amino-1-[(2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one(Cas 10212-20-1) Usage
|
Chemical Properties |
White Solid |
|
Uses |
2'-Deoxy-2'-fluorocytidine is a potent inhibitor of the subgenomic hepatitis C virus replicon in Huh-7 cells. 2'-Deoxy-2'-fluorocytidine has been shown to inhibit Borna Disease virus replication and s pread. |
InChI:InChI=1/C9H12FN3O4/c10-6-7(15)4(3-14)17-8(6)13-2-1-5(11)12-9(13)16/h1-2,4,6-8,14-15H,3H2,(H2,11,12,16)/t4-,6?,7-,8-/m1/s1
10212-20-1 Relevant articles
Synthesis and hypnotic and anti-human immunodeficiency virus-1 activities of N3-substituted 2'-deoxy-2'-fluorouridines
Sato,Utsumi,Maruyama,Kimura,Yamamoto,Richman
, p. 595 - 598 (1994)
Reaction of 9-[3,5-di-O-(tetrahydropyran...
Antisense modulation of CD40 ligand expression
-
Page/Page column 18, (2008/06/13)
Antisense compounds, compositions and me...
Modulation of DC-SIGN expression
-
Page/Page column 12, (2008/06/13)
Compounds, compositions and methods are ...
Antisense modulation of polo-like kinase expression
-
Page/Page column 18, (2008/06/13)
Antisense compounds, compositions and me...
Modulation of CEACAM1 expression
-
Page/Page column 26, (2010/02/11)
Compounds, compositions and methods are ...
10212-20-1 Process route
-
-
56287-17-3,69123-94-0,784-71-4
2'-deoxy-2'-fluorouridine

-
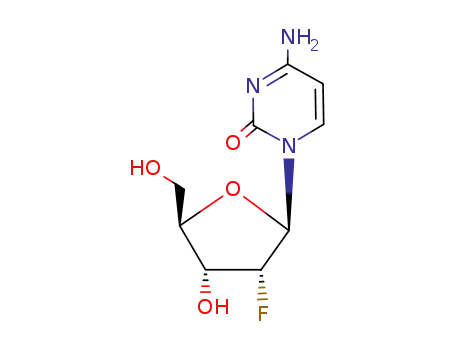
-
10212-20-1
2'-deoxy-2'-fluorocytidine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1: 92 percent / pyridine / 2 h / Ambient temperature
2: (PhO)2POCl, Et3N / acetonitrile / 1.5 h / Ambient temperature
3: 32percent aq. NH3 / dioxane / 0.33 h
4: EtNMe2 / methanol / 24 h / 50 °C
With
ammonium hydroxide; N,N-dimethyl-ethanamine; triethylamine; chlorophosphoric acid diphenyl ester;
In
1,4-dioxane; pyridine; methanol; acetonitrile;
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-
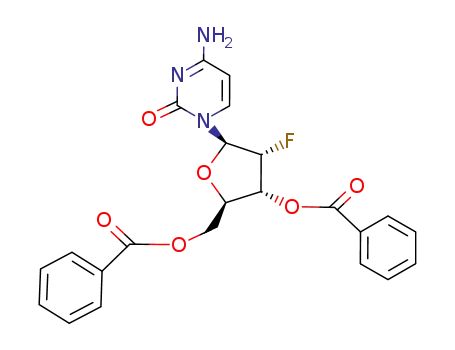
-
3',5'-Di-O-benzoyl-2'-desoxy-2'-fluorcytidin

-

-
10212-20-1
2'-deoxy-2'-fluorocytidine
| Conditions | Yield |
|---|---|
|
With
ammonium hydroxide;
at 50 ℃;
|
56% |
10212-20-1 Upstream products
-
157024-78-7
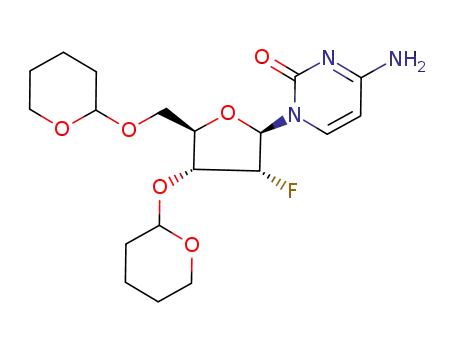
9-<2-deoxy-2-fluoro-3,5-di-O-(tetrahydropyran-2-yl)-β-D-ribofuranosyl>cytosine
-
31698-14-3
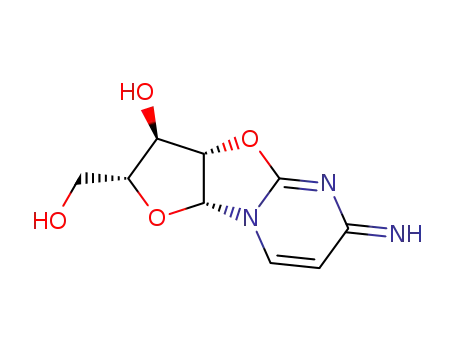
cyclocitidine
-
10212-13-2
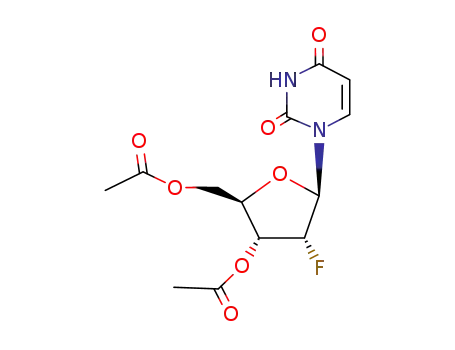
3’,5’-O-acetyl-2’-deoxy-2’-fluorouridine
-
56287-17-3

2'-deoxy-2'-fluorouridine
10212-20-1 Downstream products
-
146954-76-9
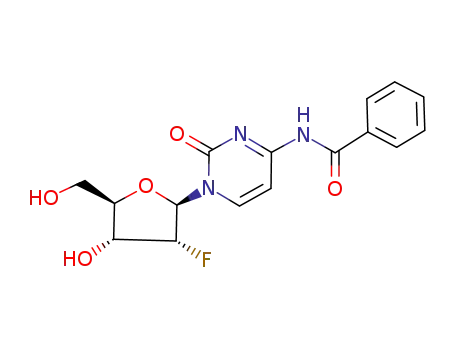
N4-benzoyl-2-deoxy-2-fluorocytidine
-
134444-47-6
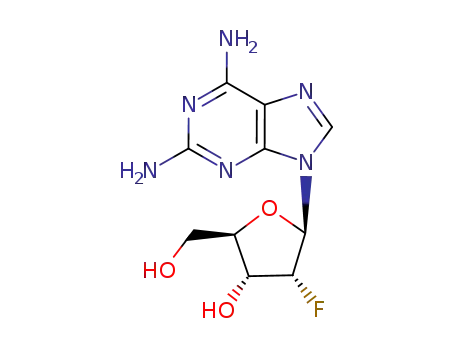
2,6-diamino-9-(2-deoxy-2-fluoro-β-D-ribofuranosyl)-9H-purine
-
80791-93-1
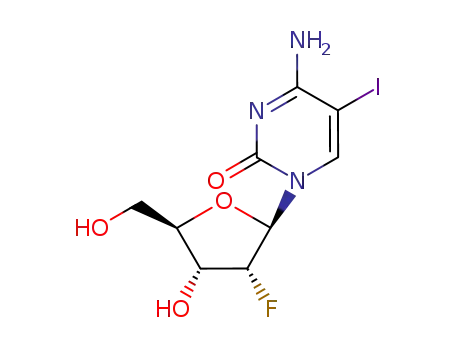
5-iodo-2'-deoxy-2'-(R)-fluoro-cytidine
-
78842-13-4
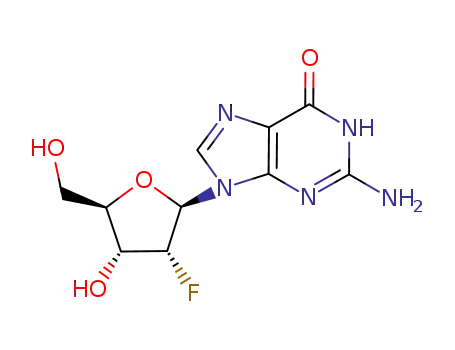
2'-deoxy-2'-fluoroguanosine
Relevant Products
-
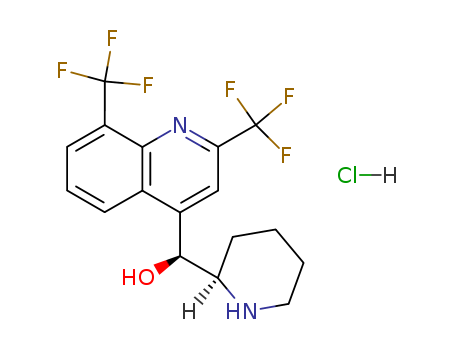
Mefloquine hydrochloride
CAS:51773-92-3
-
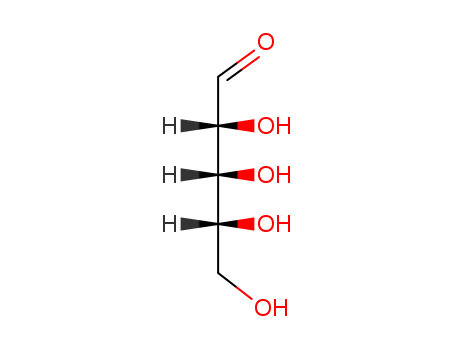
D-ribose
CAS:50-69-1
-
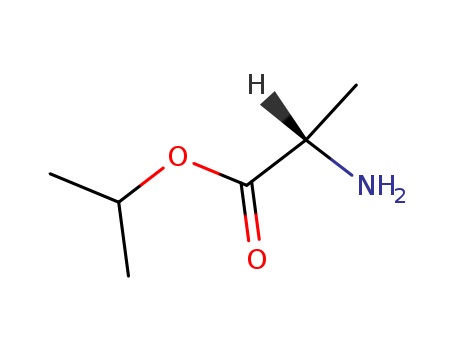
L-Alanine Isopropyl Ester Hydrochloride
CAS:39825-33-7