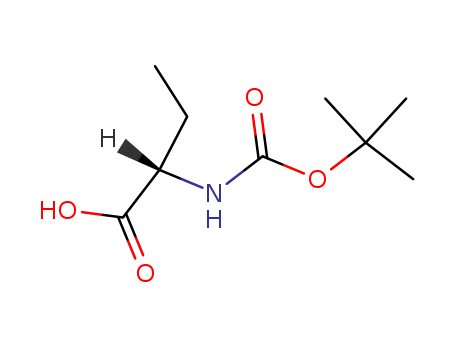
34306-42-8
- Product Name:Boc-2-aminobutanoic acid
- Molecular Formula:C9H17NO4
- Purity:99%
- Molecular Weight:203.238
Product Details;
CasNo: 34306-42-8
Molecular Formula: C9H17NO4
Appearance: White powder
Buy Quality Trustworthy Factory Supply Boc-2-aminobutanoic acid 34306-42-8 with Safe Transportation
- Molecular Formula:C9H17NO4
- Molecular Weight:203.238
- Appearance/Colour:White powder
- Vapor Pressure:2.42E-05mmHg at 25°C
- Melting Point:70-74 °C
- Refractive Index:1.46
- Boiling Point:334.5 °C at 760mmHg
- PKA:4.00±0.10(Predicted)
- Flash Point:156.1 °C
- PSA:75.63000
- Density:1.101 g/cm3
- LogP:1.76520
BOC-ABU-OH(Cas 34306-42-8) Usage
|
Chemical Properties |
Light yellow powder |
|
Uses |
It is employed as a intermediate for pharmaceutical. |
InChI:InChI=1/C9H17NO4/c1-5-6(7(11)12)10-8(13)14-9(2,3)4/h6H,5H2,1-4H3,(H,10,13)(H,11,12)/t6-/m0/s1
34306-42-8 Relevant articles
Enantioselective Deaminative Alkylation of Amino Acid Derivatives with Unactivated Olefins
Cai, Yue-Ming,Martin, Ruben,Rui, Xi-Yan,Shang, Ming,Sun, Shang-Zheng,Wang, Jia-Bao,Yao, Hong-Qing,Zhang, De-Liang
supporting information, p. 1130 - 1137 (2022/02/05)
Herein, we report the first Ni-catalyzed...
Optimization and Evaluation of Novel Antifungal Agents for the Treatment of Fungal Infection
Bahn, Yong-Sun,Cheong, Eunji,Choi, Ji Won,Jang, Bo Ko,Kim, Dahee,Kim, Hyeon Jeong,Kim, Hyeon Ji,Kim, Siwon,Lee, Dong-Gi,Lee, Jong-Seung,Lee, Kyung-Tae,Lee, Myung Ha,Lee, Ye Rim,Park, Jong-Hyun,Park, Ki Duk,Park, Sun Jun,Seo, Kyung Jin,Yeon, Seul Ki
supporting information, p. 15912 - 15935 (2021/11/10)
Due to the increased morbidity and morta...
FUNCTIONAL DERIVATIVE COMPOUNDS OF ALANINE AND PROLINE AMINO ACIDS AND PHARMACEUTICAL COMPOSITION COMPRISING SAME
-
Paragraph 0043-0044; 0057, (2019/09/15)
The present invention relates to novel f...
Biocatalytic Cascade Reaction for the Asymmetric Synthesis of L- and D-Homoalanine
Silva, Marcus V. de M.,Costa, Ingrid C. R.,de Souza, Rodrigo O. M. A.,Bornscheuer, Uwe T.
, p. 407 - 411 (2018/11/01)
Unnatural amino acids attract growing at...
34306-42-8 Process route
-
-
1492-24-6
L-2-aminobutyric acid

-
-
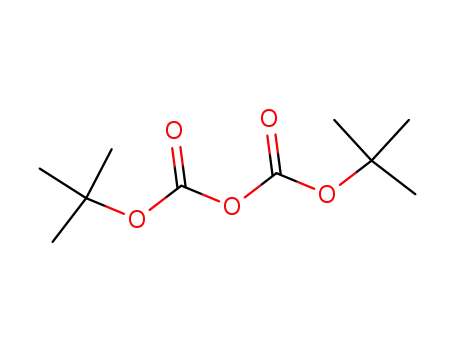
24424-99-5
di-tert-butyl dicarbonate

-
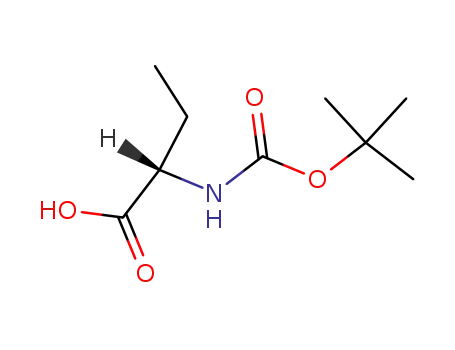
-
34306-42-8
Boc-Abu
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; sodium hydroxide;
In
water; tert-butyl alcohol;
|
100% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 20 ℃;
for 12h;
|
99% |
|
L-2-aminobutyric acid;
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 0 ℃;
for 0.166667h;
di-tert-butyl dicarbonate;
In
tetrahydrofuran; water;
at 0 - 20 ℃;
for 24h;
|
98% |
|
With
sodium hydroxide;
In
methanol;
at 0 - 20 ℃;
for 12h;
|
93% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
for 5h;
Ambient temperature;
|
91% |
|
With
sodium hydroxide;
In
methanol;
at 0 - 20 ℃;
|
91% |
|
With
sodium hydroxide;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane;
at 20 ℃;
|
|
|
L-2-aminobutyric acid; di-tert-butyl dicarbonate;
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 20 ℃;
for 4h;
With
hydrogenchloride;
In
tetrahydrofuran; water; ethyl acetate;
pH=2;
|
|
|
With
sodium carbonate;
In
1,4-dioxane; water;
|
|
|
With
potassium carbonate;
In
tetrahydrofuran; water;
for 24h;
|
-

-
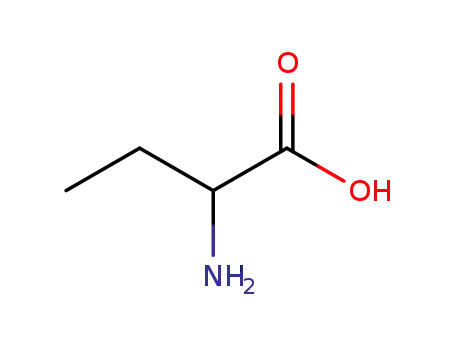
24424-99-5
di-tert-butyl dicarbonate

-
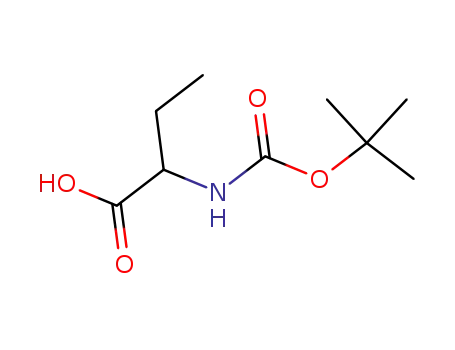
-
2835-81-6
2-aminobutanoic acid

-
-
77284-64-1,34306-42-8,45121-22-0,52881-98-8
N-(tert-butoxycarbonyl)-2(S)-aminobutyric acid
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
In
methanol; water;
at 20 ℃;
|
100% |
|
With
sodium hydroxide;
In
methanol;
at 0 - 20 ℃;
for 48h;
|
94% |
|
With
sodium hydroxide;
In
methanol;
at 20 ℃;
for 12h;
Cooling with ice;
|
93.4% |
|
With
sodium hydrogencarbonate;
In
methanol; water;
at 20 ℃;
for 36h;
Inert atmosphere;
|
83% |
34306-42-8 Upstream products
-
24424-99-5

di-tert-butyl dicarbonate
-
2835-81-6

2-aminobutanoic acid
-
40522-79-0
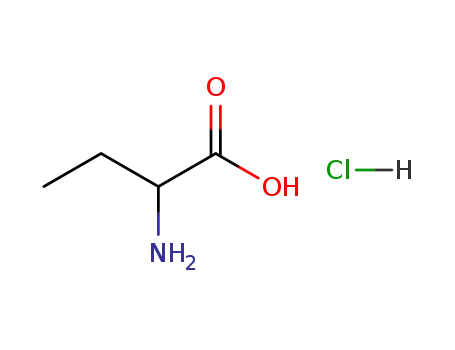
2-aminobutyric acid hydrochloride
-
1492-24-6

L-2-aminobutyric acid
34306-42-8 Downstream products
-
145280-04-2
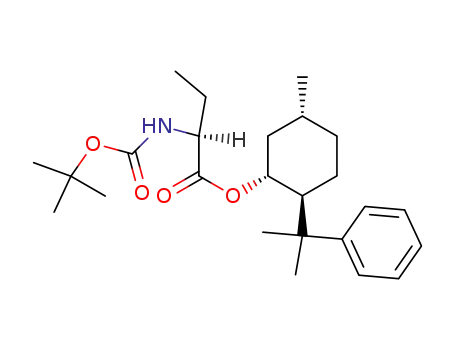
(1R,2S,5R)-2-(1-methyl-1-phenylethyl)-5-methylcyclohexyl (S)-2-<(tert-butoxycarbonyl)amino>butanoate
-
145280-03-1
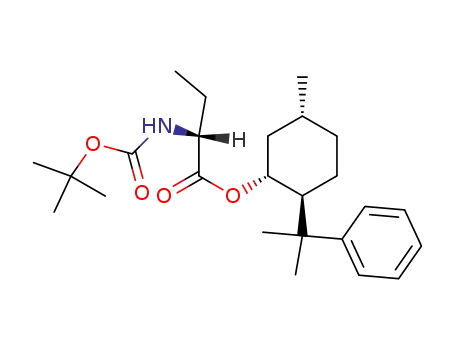
(1R,2S,5R)-2-(1-methyl-1-phenylethyl)-5-methylcyclohexyl (R)-2-<(tert-butoxycarbonyl)amino>butanoate
-
786652-81-1
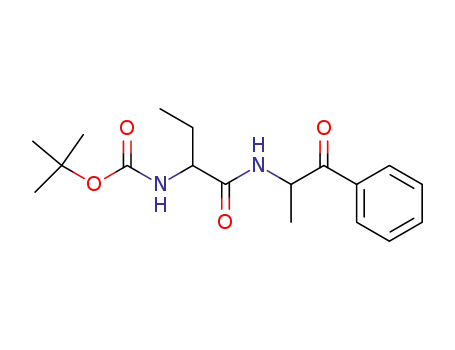
[1-(1-methyl-2-oxo-2-phenyl-ethylcarbamoyl)-propyl]carbamic acid tert-butyl ester
-
162126-54-7
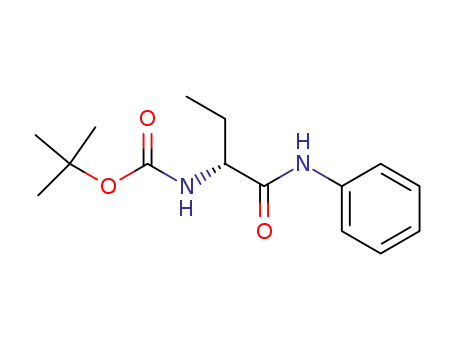
(R)-2-(t-butoxycarbonylamino)-N-phenyl-butanamide
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
2'-Fluoro-2'-deoxyuridine
CAS:784-71-4
-
D-ribose
CAS:50-69-1