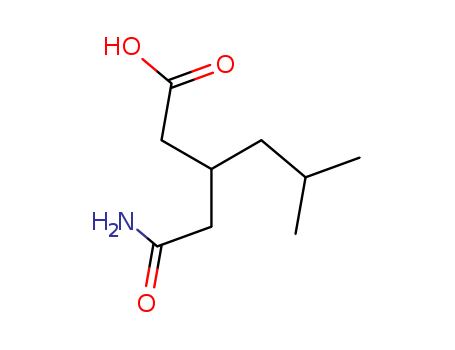
181289-15-6
- Product Name:3-Carbamoymethyl-5-methylhexanoic acid
- Molecular Formula:C9H17NO3
- Purity:99%
- Molecular Weight:187.239
Product Details;
CasNo: 181289-15-6
Molecular Formula: C9H17NO3
Reliable Quality Chinese Factory Supply 3-Carbamoymethyl-5-methylhexanoic acid 181289-15-6 with Efficient Shipping
- Molecular Formula:C9H17NO3
- Molecular Weight:187.239
- Vapor Pressure:1.39E-07mmHg at 25°C
- Melting Point:106-108 °C
- Refractive Index:1.475
- Boiling Point:401.9 °C at 760 mmHg
- PKA:4.68±0.10(Predicted)
- Flash Point:196.9 °C
- PSA:80.39000
- Density:1.08 g/cm3
- LogP:1.69910
3-Carbamoymethyl-5-methylhexanoic acid(Cas 181289-15-6) Usage
|
Physical Form |
Powder |
|
Uses |
3-?(2-?Amino-?2-?oxoethyl)?-?5-?methylhexanoic Acid is a Pregabalin, a GABA analogue used as an anticonvulsant, intermediate. |
InChI:InChI=1/C9H17NO3/c1-6(2)3-7(4-8(10)11)5-9(12)13/h6-7H,3-5H2,1-2H3,(H2,10,11)(H,12,13)
181289-15-6 Relevant articles
Development of a new synthesis approach for S-pregabalin by optimizing the preparation stages
Mansoori, Arsalan,Zahednezhad, Fahimeh,Bavili Tabrizi, Ahad,Shahbazi Mojarrad, Javid
, p. 89 - 101 (2019/09/13)
In the present study, we aimed to optimi...
Pregregregabalin intermediate mother liquor and method for recycling wastewater
-
Paragraph 0032-0049, (2020/09/23)
The invention provides a preparation met...
Y-shaped potential third-order nonlinear optical material-3-(2-amino-2-oxoethyl)-5-methyl hexanoic acid: An analysis of structural, spectroscopic and docking studies
Poojith, Nuthalapati,Potla, Krishna Murthy,Osório, Francisco A. P.,Valverde, Clodoaldo,Vankayalapati, Suneetha,Suchetan,Raja
, p. 18185 - 18198 (2020/11/13)
The present work reports an analysis of ...
Method for synthesizing optically pure (R)-3-carbamoymethyl-5-methylhexanoic acid
-
, (2019/09/14)
The invention relates to the technical f...
181289-15-6 Process route
-
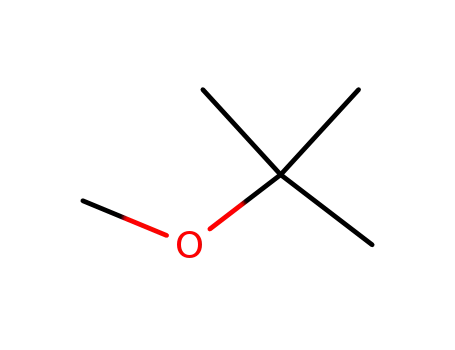
-
1634-04-4
tert-butyl methyl ether

-
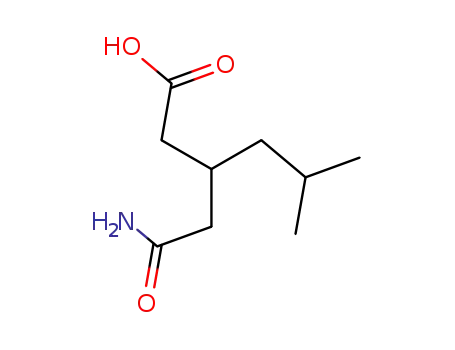
-
181289-15-6
(±)?3?(carbamoylmethyl)?5?methylhexanoic acid
| Conditions | Yield |
|---|---|
|
With
ammonium hydroxide;
In
water;
|
-
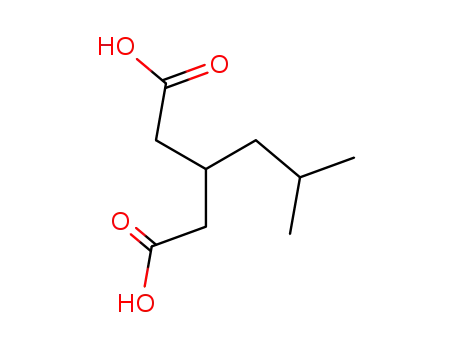
-
75143-89-4
5-methyl-3-carboxymethylhexanoic acid

-

-
181289-15-6
(±)?3?(carbamoylmethyl)?5?methylhexanoic acid
| Conditions | Yield |
|---|---|
|
With
urea;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 130 ℃;
for 3h;
Temperature;
Solvent;
|
93.5% |
|
With
pyridine; di-tert-butyl dicarbonate; ammonium bicarbonate;
In
acetonitrile;
at 5 - 20 ℃;
for 0.5h;
Solvent;
|
72.4% |
|
With
hydrogenchloride; ammonium hydroxide; acetic anhydride;
In
tert-butyl methyl ether; water; ethyl acetate;
|
|
|
5-methyl-3-carboxymethylhexanoic acid;
With
urea;
at 130 - 135 ℃;
for 12h;
With
sodium hydroxide;
In
water;
at 60 - 90 ℃;
Product distribution / selectivity;
|
|
|
Multi-step reaction with 2 steps
1.1: acetyl chloride / 3.08 h / 20 - 55 °C / Inert atmosphere
2.1: ammonia / water; tert-butyl methyl ether / 0 - 20 °C
2.2: pH 2
With
ammonia; acetyl chloride;
In
tert-butyl methyl ether; water;
|
|
|
Multi-step reaction with 3 steps
1.1: acetyl chloride / 3.08 h / 20 - 55 °C / Inert atmosphere
2.1: 1,4-diaza-bicyclo[2.2.2]octane / tert-butyl methyl ether / 2 h / -78 °C / Inert atmosphere
3.1: chloroformic acid ethyl ester; triethylamine / acetone / -20 °C / Inert atmosphere
3.2: 2 h / -20 °C
3.3: 0 °C
With
1,4-diaza-bicyclo[2.2.2]octane; chloroformic acid ethyl ester; triethylamine; acetyl chloride;
In
tert-butyl methyl ether; acetone;
|
|
|
Multi-step reaction with 2 steps
1: acetic anhydride / 20 °C
2: ammonium hydroxide / tert-butyl methyl ether / 20 °C
With
ammonium hydroxide;
In
tert-butyl methyl ether; acetic anhydride;
|
|
|
Multi-step reaction with 2 steps
1: 5 h / 160 °C
2: sodium hydroxide; water / 0.58 h / 65 °C
With
water; sodium hydroxide;
|
181289-15-6 Upstream products
-
1634-04-4

tert-butyl methyl ether
-
75143-89-4

5-methyl-3-carboxymethylhexanoic acid
-
185815-59-2
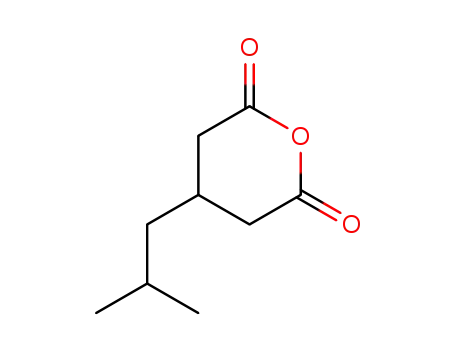
3-isobutylglutaric anhydride
-
916982-10-0
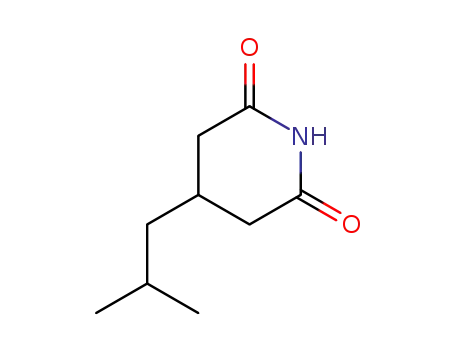
3-isobutylglutarimide
181289-15-6 Downstream products
-
1001296-60-1
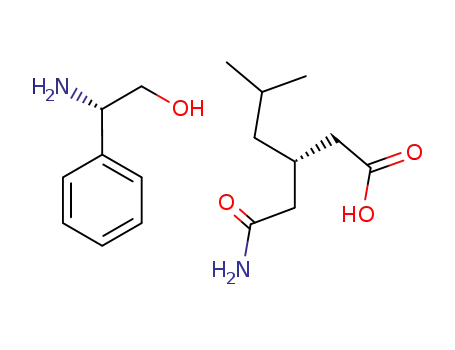
(S)-(+)-phenylglycinol salt of (S)-(+)-3-(carbamoylmethyl)-5-methylhexanoic acid
-
1001296-65-6
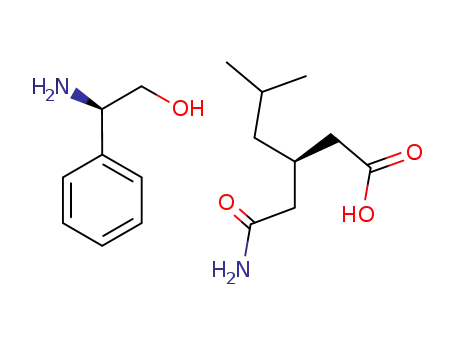
(R)-(-)-phenylglycinol salt of (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid
-
148553-50-8
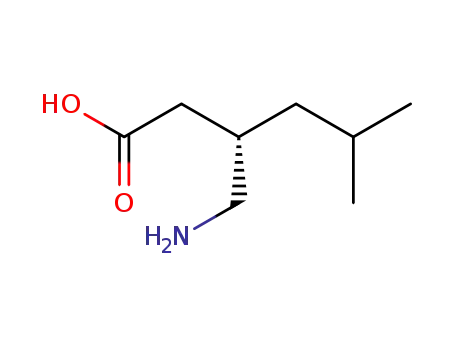
pregabilin
-
128013-69-4
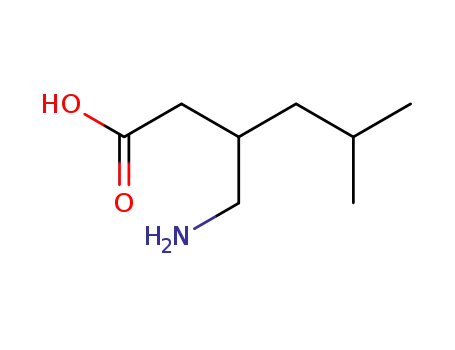
(R,S)-3-iso-butyl-4-aminobutyric acid
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
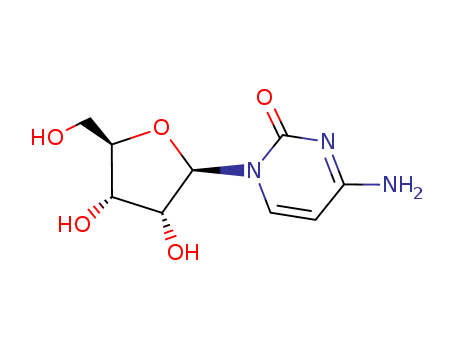
Cytidine
CAS:65-46-3
-
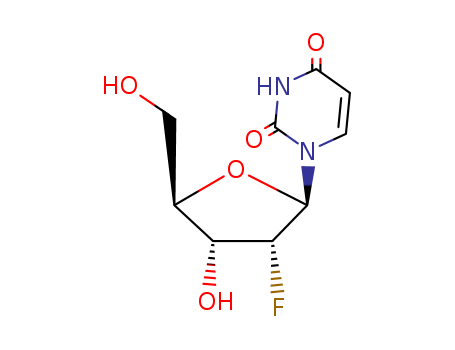
2'-Fluoro-2'-deoxyuridine
CAS:784-71-4