65-46-3
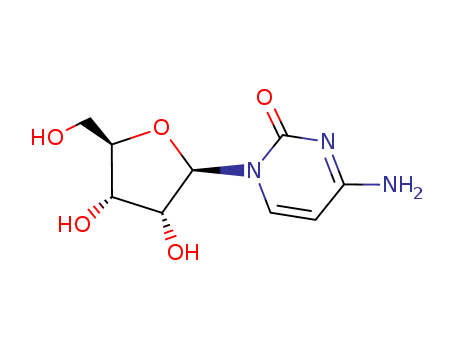
- Product Name:Cytidine
- Molecular Formula:C9H13N3O5
- Purity:99%
- Molecular Weight:243.219
Product Details;
CasNo: 65-46-3
Molecular Formula: C9H13N3O5
Appearance: White crystalline powder
Wholesale Factory Supply High Purity Cytidine 65-46-3 with Cheap Price
- Molecular Formula:C9H13N3O5
- Molecular Weight:243.219
- Appearance/Colour:White crystalline powder
- Vapor Pressure:3.5E-14mmHg at 25°C
- Melting Point:210-217 °C
- Refractive Index:34 ° (C=0.7, H2O)
- Boiling Point:545.7 °C at 760 mmHg
- PKA:4.22, 12.5(at 25℃)
- Flash Point:283.8 °C
- PSA:130.83000
- Density:1.89 g/cm3
- LogP:-1.98180
Cytidine(Cas 65-46-3) Usage
|
Description |
Cytidine is a pyrimidine nucleoside that is composed of the pyrimidine base cytosine attached to the sugar ribose. As a constituent of RNA, cytidine pairs with guanine, and it is a precursor of uridine. Cytidine can incur several modifications in mRNA, including methylation and acetylation, that function to regulate translation. Cytidine can also be formylated to 5-formylcytidine in mitochondrial methionine transfer RNA (tRNAMet). |
|
Chemical Properties |
Cytidine is a component of RNA. It is a white water-soluble solid. which is only slightly soluble in ethanol.There are a variety of cytidine analogs with potentially useful pharmacology. |
|
Uses |
Cytidine is a nucleoside molecule that is formed when cytosine is attached to a ribose ring, cytidine is a component of RNA. It was isolated from yeast nucleic acid. It can increases cell membrane phospholipids. Cytidine is used to synthesize enzyme inhibitors, antiviral agents, and anticancer agents. And also used as constituent of nucleic acids. |
|
Definition |
ChEBI: cytidine is a pyrimidine nucleoside in which cytosine is attached to ribofuranose via a beta-N(1)-glycosidic bond. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It derives from a cytosine.The derivednucleotides, cytidine mono-, di-, andtriphosphate (CMP, CDP, and CTP respectively)participate in various biochemicalreactions, notably inphospholipid synthesis. |
|
Application |
Cytidine has been used as a non-essential aminoacid component of minimal essential medium (MEM) to analyse interspecies somatic cell nucleus transfer (iSCNT)-derived blastocysts. It has also been used as a supplement in the culture medium of HeLa cells, to study the effects of cytidine addition on rods and rings (RR) induced by glutamine deprivation.Cytidine has been used as a component to prepare ribonucleoside stock solution to assess its effects on the anaerobic growth of several Bacillus mojavensis strains. |
|
General Description |
White crystalline powder. |
|
Air & Water Reactions |
Cytidine is hygroscopic. Water soluble. |
|
Reactivity Profile |
Cytidine is incompatible with strong oxidizing agents. . Will react as a weak base. |
|
Fire Hazard |
Flash point data for Cytidine are not available; however, Cytidine is probably combustible. |
|
Purification Methods |
Cytidine crystallises from 90% aqueous EtOH. It has also been converted to sulfate by dissolving (~200mg) in a solution of EtOH (10mL) containing H2SO4 (50mg), whereby the salt crystallises out. It is collected, washed with EtOH and dried for 5hours at 120o/0.1mm. The sulfate has m 225o. The free base is obtained by shaking the salt solution with a weak ion-exchange resin, filtering, evaporating and recrystallising the residue from EtOH as before. [Fox & Goodman J Am Chem Soc 73 3256 1956, Fox & Shugar Biochim Biophys Acta 9 369 1952; see Prytsas & Sorm in Synthetic Procedures in Nucleic Acid Chemistry (Zorbach & Tipson Eds) Vol 1 404 1973.] [Beilstein 25 III/IV 3667.] |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16)/t4-,6+,7+,8+/m1/s1
65-46-3 Relevant articles
-
Hayes
, p. 1184,1186 (1960)
-
-
Wright et al.
, p. 1898 (1951)
-
-
Bamann,Trapmann
, p. 237,239 (1954)
-
Meteorite-catalyzed intermoleculartrans-glycosylation produces nucleosides under proton beam irradiation
Bizzarri, Bruno Mattia,Fanelli, Angelica,Kapralov, Michail,Krasavin, Eugene,Saladino, Raffaele
, p. 19258 - 19264 (2021/06/03)
Di-glycosylated adenines act as glycosyl...
Conversion of: N- acyl amidines to amidoximes: A convenient synthetic approach to molnupiravir (EIDD-2801) from ribose
Ahmed, Ajaz,Ahmed, Qazi Naveed,Mukherjee, Debaraj
, p. 36143 - 36147 (2021/12/04)
An efficient method is described for the...
DIVERSE AND FLEXIBLE CHEMICAL MODIFICATION OF NUCLEIC ACIDS
-
Paragraph 0124-0126; 0131; 0134, (2020/05/12)
The present invention provides a method ...
Dehalogenation of Halogenated Nucleobases and Nucleosides by Organoselenium Compounds
Mondal, Santanu,Mugesh, Govindasamy
, p. 1773 - 1780 (2019/01/10)
Halogenated nucleosides, such as 5-iodo-...
65-46-3 Process route
-
highly polymerized dsDNA from salmon testes
-
highly polymerized dsDNA from salmon testes

-
-
108-28-1
protoanemonin

-
-
98-01-1
furfural

-
-
542-78-9
Malondialdehyde

-
-
N-oxycarbonylmethyl-5-methylene-Δ3-pyrrolin-2-one

-
-
118-00-3
G

-
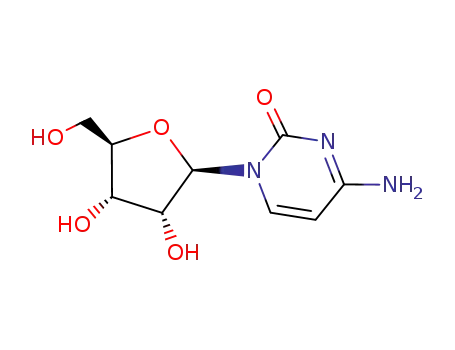
-
65-46-3
CYTIDINE

-
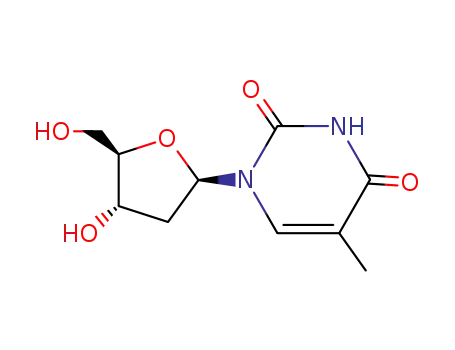
-
50-89-5,28854-96-8,3545-96-8,50-88-4
thymidine

-
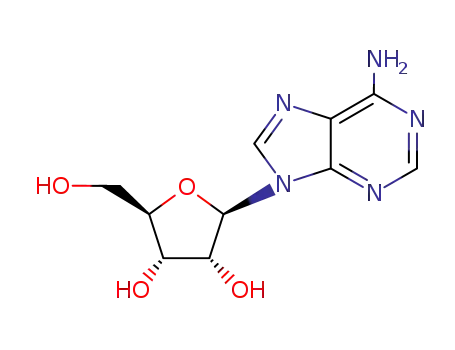
-
58-61-7
adenosine
| Conditions | Yield |
|---|---|
|
With
carbonatopentamminecobalt(III) nitrate hemihydrate;
In
aq. phosphate buffer;
at 20 ℃;
for 0.133333h;
pH=6.9;
UV-irradiation;
|
-
5'-CGGCUXUUAACCGA-3', X=2-deoxouridine
-
5'-CGGCUXUUAACCGA-3', X=2-deoxouridine

-

-
118-00-3
G

-
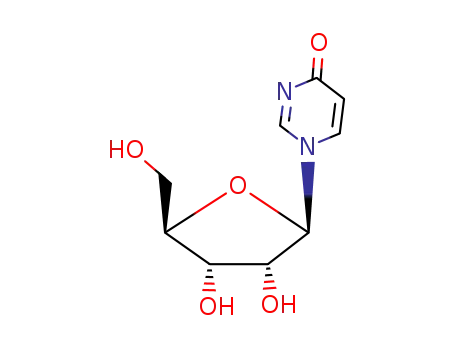
-
21052-20-0
1-(β-D-ribofuranosyl)-4-pyrimidinone

-
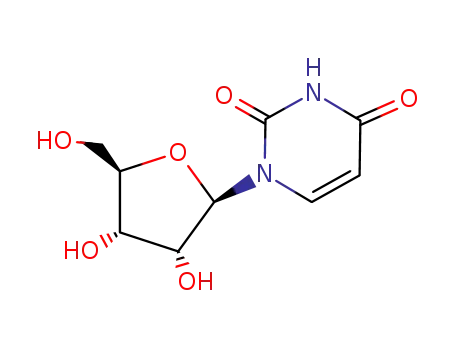
-
58-96-8
uridine

-

-
65-46-3
CYTIDINE

-

-
58-61-7
adenosine
| Conditions | Yield |
|---|---|
|
5'-CGGCUXUUAACCGA-3', X=2-deoxouridine;
With
1U P1 nuclease;
In
aq. buffer;
at 37 ℃;
for 16h;
pH=7;
Enzymatic reaction;
With
2U alkaline phosphatase;
In
aq. buffer;
at 37 ℃;
for 1h;
Enzymatic reaction;
|
65-46-3 Upstream products
-
27391-03-3
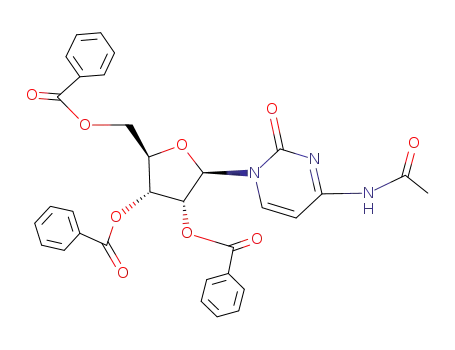
N4-acetyl-O2',O3',O5'-tribenzoyl-cytidine
-
15049-50-0
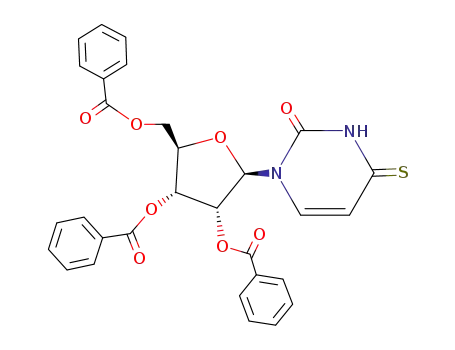
1-(2',3',5'-tri-O-benzoyl-β-D-ribofuranosyl)-4-thiouracil
-
6974-32-9
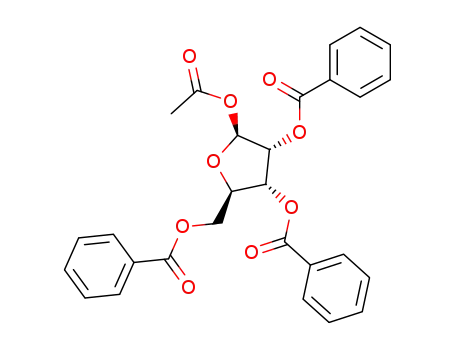
1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose
-
33304-87-9
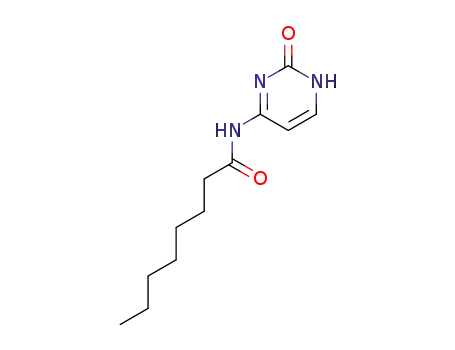
N4-octanoylcytosine
65-46-3 Downstream products
-
2536-99-4
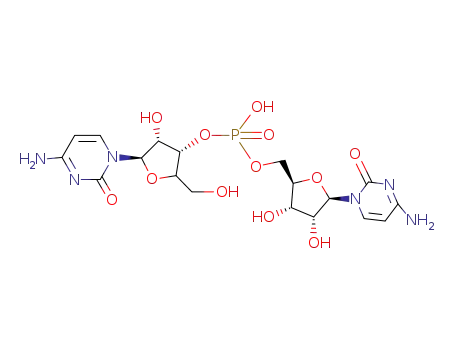
[3']cytidylic acid cytidin-5'-yl ester
-
4785-04-0
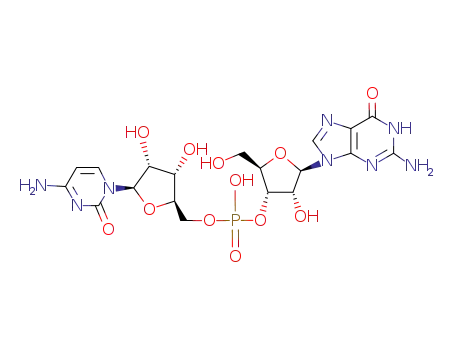
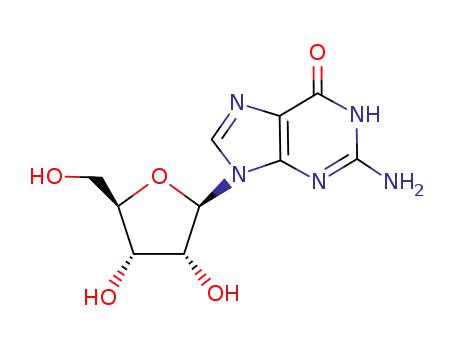
Phosphoric acid (2R,3S,4R,5R)-5-(2-amino-6-oxo-1,6-dihydro-purin-9-yl)-4-hydroxy-2-hydroxymethyl-tetrahydro-furan-3-yl ester (2R,3S,4R,5R)-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethyl ester
-
22596-01-6
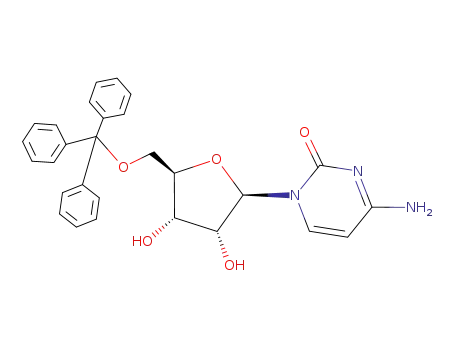
5'-O-(triphenylmethyl)cytidine
-
3257-72-5
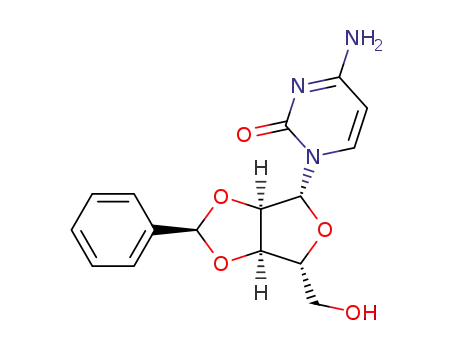
O2',O3'-((R)-benzylidene)-cytidine
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
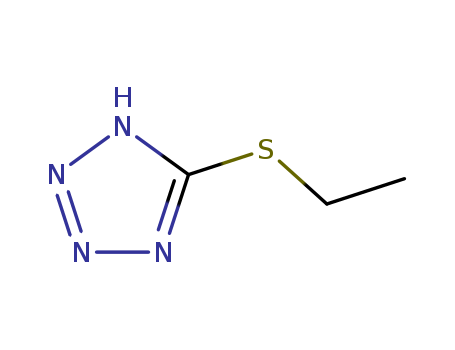
5-(Ethylthio)-1H-tetrazole
CAS:89797-68-2
-
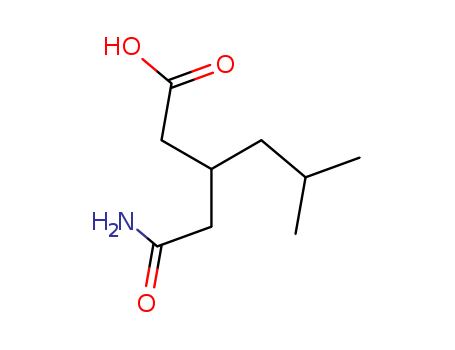
3-Carbamoymethyl-5-methylhexanoic acid
CAS:181289-15-6