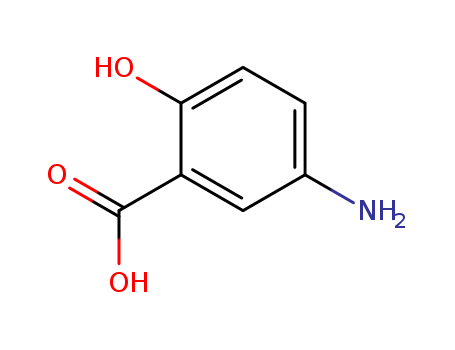
89-57-6
- Product Name:5-Aminosalicylic acid
- Molecular Formula:C7H7NO3
- Purity:99%
- Molecular Weight:153.137
Product Details;
CasNo: 89-57-6
Molecular Formula: C7H7NO3
Appearance: Off-white crystals
Reliable Quality Reputable Factory Supply 5-Aminosalicylic acid 89-57-6 In Bulk Supply
- Molecular Formula:C7H7NO3
- Molecular Weight:153.137
- Appearance/Colour:Off-white crystals
- Vapor Pressure:3.01E-07mmHg at 25°C
- Melting Point:275-280 °C (dec.)(lit.)
- Refractive Index:1.5500 (estimate)
- Boiling Point:403.9 °C at 760 mmHg
- PKA:2.74, 5.84(at 25℃)
- Flash Point:198.1 °C
- PSA:83.55000
- Density:1.491 g/cm3
- LogP:1.25380
5-Aminosalicylic acid(Cas 89-57-6) Usage
|
Overview |
Mesalazine is an anti-inflammatory agent, structurally related to the salicylates, which is active in inflammatory bowel disease. It is considered to be the active moiety of sulphasalazine. A study of the therapeutic properties of sulfasalazine and its constituents[mesalazine (5-amino salicylic acid, 5-ASA) and sulfapyridine] indicated that mesalazine is the therapeutically active component, while sulfapyridine acts as an inert carrier molecule to facilitate delivery to the colon.[2] This discovery, coupled with the implication of sulfapyridine in most of the adverse events associated with sulfasalazine treatment,[3] led to the development of Mesalazine as a pure therapeutic entity. Mesalazine (fig. 1) is believed to exert its effects via topical actions in the gut lumen. However, orally administered unconjugated mesalazine is extensively absorbed from the proximal small bowel,[4] and alternative oral dosage formulations have been developed to facilitate the delivery of mesalazine to more distal sites of inflammation. These include microgranules of mesalazine coated with a semipermeable ethylcellulose membrane (Pentasa?), mesalazine encased within a pH-dependent acrylic resin (pH-dependent delayed-release preparations: Salofalk?, Claversal?, Mesasal?, Asacol?), or conjugation of mesalazine via an azo bond to an inert carrier (balsalazide) or to another mesalazine molecule (olsalazine). In each case the properties of the delivery system dictate the site of mesalazine release. Figure 1 the chemical structure of Mesalazine |
|
Indication |
It is used for the treatment of active ulcerative proctitis. |
|
Mode of action |
The pathogenesis of IBD and hence the mechanism by which mesalazine exerts its therapeutic effects in this disease remain elusive. However, lipid mediators[leukotrienes (LT), prostaglandins (PG), platelet-activating factor (PAF)], cytokines[including interleukins (IL), interferon-(IFN)γ and tumour necrosis factor-(TNF)α] and reactive oxygen species have been implicated in the nonspecific inflammation and tissue damage characteristic of IBD.[5-7] The modulation of these molecules by mesalazine may underlie the therapeutic effects of the drug.[8-11] Numerous in vitro studies have investigated the effects of mesalazine on inflammatory processes in colonic epithelial cell lines or biopsy specimens from patients with active ulcerative colitis or with normal colons. Mesalazine also appears to reduce in vitro levels of LTC4, 5-hydroxyeicosatetraenoic acid (HETE), 11-, 12-, 15-HETE, PGD2 and platelet-activating factor. In addition to inhibiting interferon (IFN)-γ binding, mesalazine reduced IFNγ-induced cell permeability and expression of the HLA-DR product of the major histocompatibility complex in colonic epithelial cell lines. Recent evidence suggests that mesalazine reverses the antiproliferative effects of tumour necrosis factor-(TNF)α and inhibits TNFα signalling events in intestinal cells. Mesalazine may also reduce interleukin (IL)-1/1β and IL-2 production. A variety of data from experimental work, animal studies and preliminary clinical trials strongly suggest that mesalazine may have antineoplastic and potentially prophylactic (chemo-preventive) properties, which are comparable with those found with aspirin and other NSAIDs. Mesalazine shares similar molecular targets, interfering with inflammation, proliferation and ? or apoptosis, as aspirin and other NSAIDs. This can be explained by the close molecular similarity of mesalazine and aspirin, in which the former differs only in structure by the presence of an amino group at position 5 of the benzene ring. Recent experimental and preliminary clinical work has demonstrated that mesalazine may have in vitro and in vivo inhibitory properties comparable to other NSAIDs.[12-14] Reversible inhibition of COX-1 and COX-2, NF-kB activation, MAP kinases and Bcl-2 by mesalazine, was found in experiments using different cell systems including lymphocytes, polymorphonuclear leucocytes (PMNLs) and cultures from normal and neoplastic cell lines of animal and human origin.[15] In contrast to aspirin, which was shown to inhibit COX irreversibly, mesalazine (and other NSAIDs) inhibit COX and other steps (e.g. Bcl-2) reversibly. The molecular details for the majority of these reactions are only partly known, but recent work has shed light on some of these. Thus, inhibition of NF-kB activation is most likely to be mediated by inhibition of IkB degradation, the inhibitory unit of the NF-kB complex. It is worth noting that mesalazine has rather unspecific COX inhibitory properties with no preference for COX-2. |
|
Pharmacokinetics |
After a single oral dose of prolonged-release mesalazine 250mg to volunteers, the median lag time (tlag) to the first detectable plasma concentration of mesalazine was 45 minutes (range 15 to 150). A maximum plasma concentration (Cmax) of 0.6 μmol/L (range 0.4 to 1.4) was recorded 240 minutes (tmax; 90 to 300) after dose administration. Corresponding values for acetyl mesalazine were: tlag 22 minutes (15 to 45), Cmax 2.9 μmol/L (1.6 to 3.4) and tmax 105 minutes (60 to 300).[16] The plasma concentration-time profile following a single oral dose of prolonged-release mesalazine 1g to healthy volunteers was consistent with a continuous release of drug throughout the gastrointestinal tract. Plasma concentrations peaked at 0.53 mg/L 4 hours after administration, declined rapidly to 0.03 mg/L at 12 hours, then remained fairly constant over the next 24 hours before resuming the final decline, becoming undetectable 60 hours after administration. The area under the plasma concentrationtime curve (AUC) for mesalazine was 4.37-mg/L ? h. Little is known about the distribution of prolonged-release mesalazine. In 9 pregnant women with IBD who were receiving prolonged-release mesalazine 0.5 to 3 g/day, low concentrations (approximate values from graph) of mesalazine and acetylmesalazine were measured in maternal (≤0.5 and ≤7.5 μmol/L) and fetal plasma (≤0.25 and ≤18 μmol/L). In 2 patients, low concentrations of mesalazine were detected in breast milk. Mean acetyl mesalazine concentrations in breast milk were 4.4 to 47.5 μmol/L.[18, 19] Mesalazine is primarily metabolized by acetylation in the gut wall and the liver, forming the therapeutically inert metabolite acetyl mesalazine. Both the parent compound and the metabolite are excreted in the urine.[20] After a single oral administration of prolonged-release mesalazine 0.25g in 6 volunteers, the apparent mean elimination half-life of acetyl mesalazine was 802 minutes (range 608 to 993). Determination of the terminal half-life of mesalazine was not possible because of low plasma concentrations.[16] After oral administration of prolonged-release mesalazine 1.5 to 4 g/day to volunteers, excretion of unchanged mesalazine accounted for 8 to 12% of the daily dose. Total urinary excretion of mesalazine plus acetyl mesalazine was 29 to 53%.[21,22,23] In volunteers, renal clearance of acetyl mesalazine was 12 L/h (201 ml/min) at steady state.[21] In a 7-day study of 15 patients with ulcerative colitis, daily urinary excretion of mesalazine and acetyl mesalazine was higher with prolonged-release mesalazine (1.5 g/day) and pH-dependent delayed-release mesalazine (Asacol ?, 1.2 g/day) than with olsalazine (1 g/day).[17] |
|
Adverse reactions and toxicity |
In an 8-week randomized trial of prolonged release mesalazine 1, 2 and 4 g/day or placebo in patients (n = 314) with ulcerative colitis, 16% of patients receiving active drug experienced treatment-related adverse events, compared with 22% of patients in the placebo group. No dose-response relationship was observed. In total, 5%, 9% and 7% of patients in the 1, 2 and 4 g/day dosage groups discontinued therapy because of treatment-related or unrelated events, compared with 12% of placebo treated patients. The most common treatment limiting adverse events were diarrhoea, abdominal pain, fever and melaena.[24] In another 16-week study, the most common adverse events considered to be related to prolonged-release mesalazine treatment were nausea and/or vomiting (7.4 vs 3.7% in the placebo group), headache (5.2 vs 3.7%) and abdominal pain (4.3 vs 5.0%).[26] In a 12-month study involving 205 patients with ulcerative colitis, adverse events necessitating withdrawal occurred in 14% and 33% (2% and 6% considered to be treatment-related) of patients receiving prolonged-release mesalazine 4 g/day and placebo, respectively. Treatment-related adverse events (most commonly nausea 2.9%, abdominal pain 1.9% and dyspepsia 1.9%) were experienced in 6.8% of patients receiving prolonged-release mesalazine. In contrast, 11.8% of patients in the placebo group experienced adverse events related to therapy.[25] In a non-comparative study in 467 patients with Crohn’s disease who received prolongedrelease mesalazine at dosages up to 4 g/day for a median of 14 months, 12%of patients discontinued because of treatment-related adverse events, of which the most commonly reported were diarrhoea (4.3%), abdominal pain (3.6%) and dyspepsia (3.1%).[27] |
|
Description |
5-Aminosalicylic acid (5-ASA) is a metabolite and potential pharmacologically active component of sulphasalazine, a drug used in the treatment of Crohn’s disease and ulcerative colitis. However, the mechanism by which this drug works has not been established. In whole blood assays, 5-ASA proves to be a weak, non-selective inhibitor of both COX-1 and COX-2 with IC50 values of 410 and 61 μM, respectively. In ionophore-stimulated colonic mucosal cells, 1 mM 5-ASA does not inhibit prostaglandin E2 (PGE2) production, but does reduce leukotriene B4 (LTB4) synthesis approx. 50%. In ionophore-stimulated human leukocytes, 400 μM 5-ASA reduces LTB4 production approximately 20%. 5-ASA does not inhibit 15-hydroxy PGDH at concentrations up to 50 μM. |
|
Originator |
Radcliffe Infirmary (United Kingdom) |
|
Uses |
In manufacture of light-sensitive paper, azo and sulfur dyes. |
|
Definition |
ChEBI: A monohydroxybenzoic acid that is salicylic acid which is substituted by an amino group at the 5-position. |
|
Manufacturing Process |
Procedure A: To 5-nitrosalicylic acid potassium salt (55 g, 246 mmol) dissolved in water (200 mL) was added potassium hydroxide pellets to reach pH 11.5. To this solution 2 g of Raney nickel were added. The mixture was heated-up to reflux and hydrazine hydrate (40 mL, 80% in water, 64 mmol) was added dropwise during 3-4 hrs. The reflux was maintened until HPLC showed the disappearance of the starting material and the complete reduction of 5-nitrosalicylic acid (3-4 hrs). The hot mixture was filtered under nitrogen and the solution was collected. The solution was cooled to 40°C and the pH was adjusted to 2.3 by addition of 35% HCl aqueous solution. The precipitation of 5-aminosalicylic acid occurred. The solution was cooled at 0°C, and after standing at this temperature for 2 hr, the precipitate was filtered, washed with water, and dried at 60-70°C. 5-Aminosalicylic acid was obtained in 89% yield. Procedure B: To 5-nitrosalicylic acid potassium salt (55 g, 246 mmol) dissolved in water (200 mL) was added potassium hydroxide pellets to reach pH 11.5. The solution was charged in a stainless steel autoclave and 2 g of Raney nickel are added. Hydrogen was introduced into the autoclave reaching a pressure of 8 atm. The mixture was heated-up to 100°C. The temperature was maintained until HPLC-test 5-aminosalicylic acid showed the disappearance of the starting material and the complete reduction of 5- aminosalicylic acid (6-8 hrs). Hydrogen was purged and replaced by nitrogen. The hot mixture was filtered under nitrogen, the filtrate was cooled to 40°C, and the pH was adjusted to 2.3 by addition of 35% HCl aqueous solution. The precipitation of the 5-aminosalicylic acid occurred. The solution was cooled at 0°C, and after standing at this temperature for 2 hr, the precipitate was filtered, washed with ion depleted water, and dried at 60-70°C. |
|
Brand name |
SALOFALK |
|
Therapeutic Function |
Antibacterial |
|
General Description |
Odorless white to pinkish crystals or purplish-tan powder. Aqueous solutions acidic (pH approximately 4.1 at 0.8 mg/L water) . |
|
Air & Water Reactions |
Sensitive to moisture. Water insoluble. |
|
Reactivity Profile |
5-Aminosalicylic acid is incompatible with acids, acid chlorides, acid anhydrides, chloroformates and strong oxidizers. |
|
Fire Hazard |
Flash point data for 5-Aminosalicylic acid are not available; however, 5-Aminosalicylic acid is probably combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Biochem/physiol Actions |
5-Aminosalicylic acid (5-ASA) is a first-line medicine, used to treat inflammatory bowel diseases like ulcerative colitis (UC). It has a high-efficiency rate in maintenance and induction of remission. 5-ASA is an active component of sulfasalazine and also consists of the carbohydrate polymer, inulin. It might exhibit anti-oxidant activity to lessen tissue injury. 5-ASA is vital for the prevention of T cell activation and proliferation. It negatively regulates cyclooxygenase and lipoxygenase pathways and lowers the formation of prostaglandins and leukotrienes. 5-ASA stimulates the membranous expression of E-cadherin and boosts intercellular adhesion. |
|
Safety Profile |
Poison by intraperitoneal route.Moderately toxic by ingestion. Human systemic effects byingestion: hypermotility, diarrhea, dermatitis, increasedbody temperature. When heated to decomposition it emitstoxic fumes of NOx. |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
The absorbed part of mesalazine is almost completely acetylated in the gut wall and in the liver to acetyl-5- aminosalicylic acid. The acetylated metabolite is excreted mainly in urine by tubular secretion, with traces of the parent compound. |
|
Purification Methods |
It crystallises as needles from H2O containing a little NaHSO3 to avoid aerial oxidation to the quinone-imine. The Me ester gives needles from *C6H6, m 96o, and the hydrazide has m 180-182o (from H2O). [Fallab et al. Helv Chim Acta 34 26 1951, Shavel J Amer Pharm Assoc 42 402 1953, Beilstein 14 IV 2058.] |
InChI:InChI=1/C7H7NO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,8H2,(H,10,11)/p-1
89-57-6 Relevant articles
Advanced Real-Time Process Analytics for Multistep Synthesis in Continuous Flow**
Sagmeister, Peter,Lebl, René,Castillo, Ismael,Rehrl, Jakob,Kruisz, Julia,Sipek, Martin,Horn, Martin,Sacher, Stephan,Cantillo, David,Williams, Jason D.,Kappe, C. Oliver
supporting information, p. 8139 - 8148 (2021/03/01)
In multistep continuous flow chemistry, ...
Functional Group-Directed Photochemical Reactions of Aromatic Alcohols, Amines, and Thiols Triggered by Excited-State Hydrogen Detachment: Additive-free Oligomerization, Disulfidation, and C(sp2)-H Carboxylation with CO2
Abe, Kanae,Nakada, Akinobu,Matsumoto, Takeshi,Uchijyo, Daiki,Mori, Hirotoshi,Chang, Ho-Chol
, p. 959 - 969 (2020/12/23)
Exploring new types of photochemical rea...
Method for synthesizing mesalazine
-
Paragraph 0011; 0048-0052, (2020/08/25)
A method for synthesizing mesalazine is ...
One-pot catalytic synthesis method of 5-aminosalicylic acid
-
Paragraph 0013; 0026-0049, (2020/08/27)
According to the invention, an aniline s...
89-57-6 Process route
-
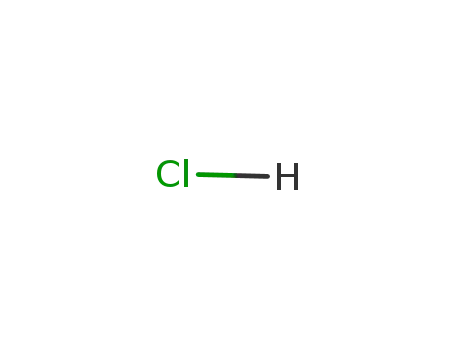
-
7647-01-0,15364-23-5
hydrogenchloride

-
![2-hydroxy-5-[1]naphthylazo-benzoic acid](/upload/2024/4/127e6430-a6db-48f1-9c18-7430c19db12f.png)
-
34898-05-0
2-hydroxy-5-[1]naphthylazo-benzoic acid

-
-
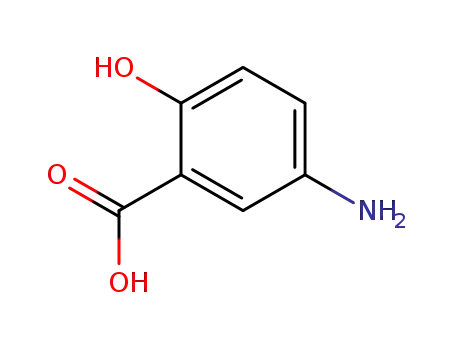
89-57-6
5-Aminosalicylic Acid

-
-
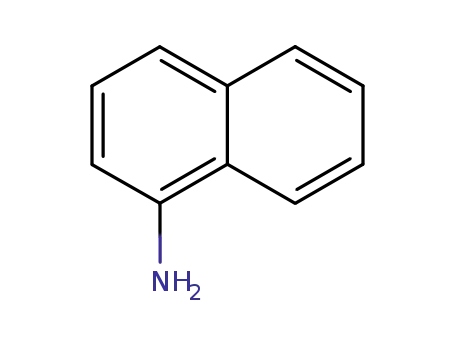
134-32-7
1-amino-naphthalene
| Conditions | Yield |
|---|---|
|
|
-
-
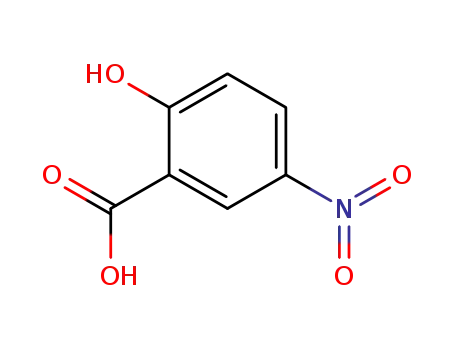
96-97-9
5-nitrosalicylic acid

-

-
89-57-6
5-Aminosalicylic Acid

-
-
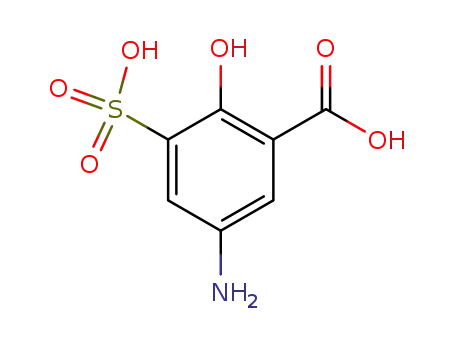
6201-87-2
5-amino-2-hydroxy-3-sulfobenzoic acid
| Conditions | Yield |
|---|---|
|
|
89-57-6 Upstream products
-
21460-89-9
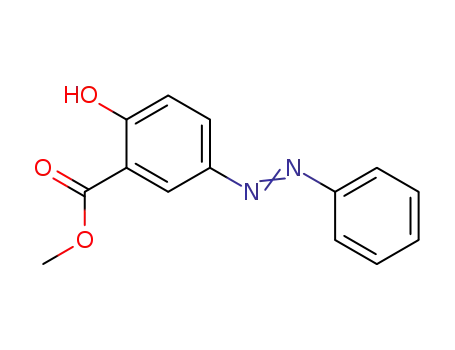
2-hydroxy-5-phenylazobenzoic acid methyl ester
-
96-97-9

5-nitrosalicylic acid
-
51-78-5
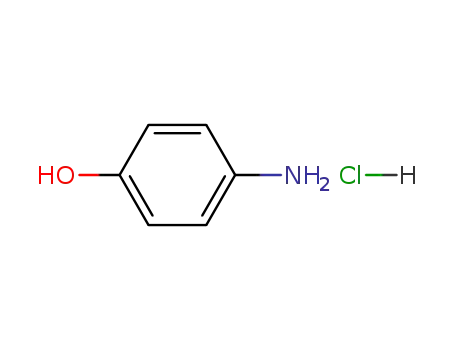
p-aminophenol hydrochloride
-
15719-64-9
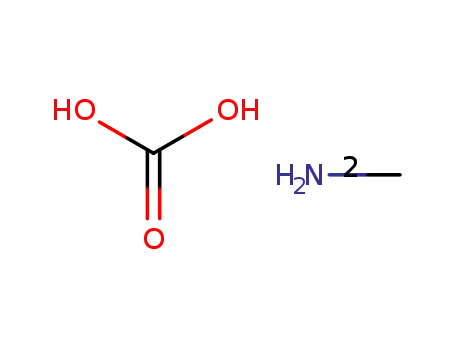
methylammonium carbonate
89-57-6 Downstream products
-
500301-76-8
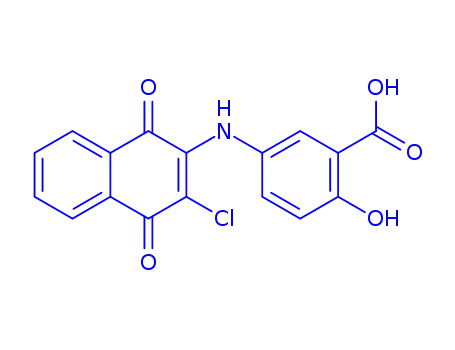
5-(3-chloro-1,4-dioxo-1,4-dihydro-[2]naphthylamino)-2-hydroxy-benzoic acid
-
42753-75-3
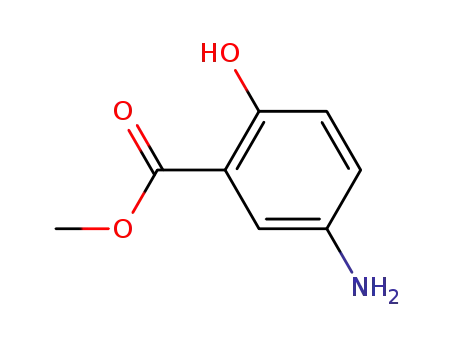
methyl 5-amino-2-hydroxybenzoate
-
53185-69-6
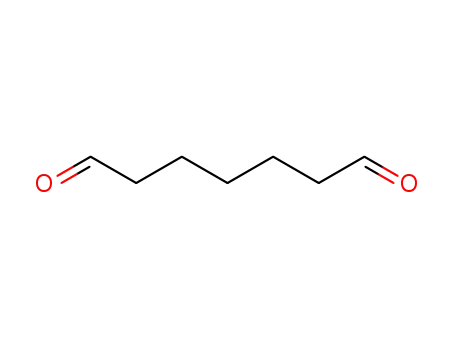
pimelaldehyde
-
119-30-2
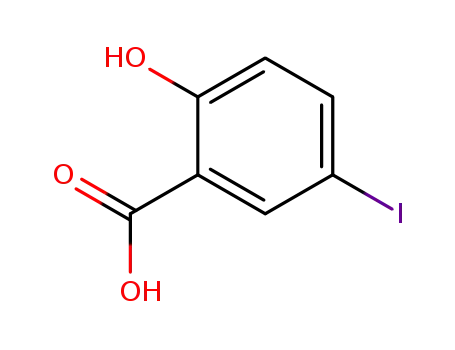
2-hydroxy-5-iodobenzoic acid
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
N-acetyl-L-tyrosine
CAS:537-55-3
-
Pseudouridine
CAS:1445-07-4