1445-07-4
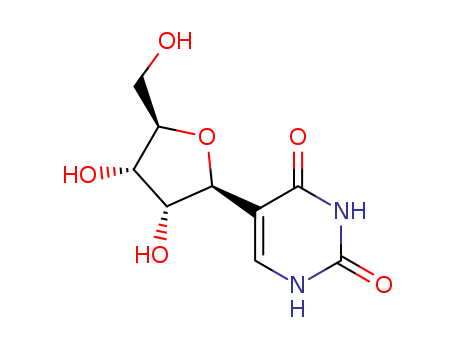
- Product Name:Pseudouridine
- Molecular Formula:C9H12N2O6
- Purity:99%
- Molecular Weight:244.204
Product Details;
CasNo: 1445-07-4
Molecular Formula: C9H12N2O6
Factory Supply 99% Pure Pseudouridine 1445-07-4 with Cheap Price
- Molecular Formula:C9H12N2O6
- Molecular Weight:244.204
- Vapor Pressure:3.63E-15mmHg at 25°C
- Melting Point:222 °C
- Refractive Index:1.623
- Boiling Point:598.4 °C at 760 mmHg
- PKA:8.52±0.10(Predicted)
- Flash Point:315.7 °C
- PSA:135.64000
- Density:1.641 g/cm3
- LogP:-2.78280
BETA-PSEUDOURIDINE(Cas 1445-07-4) Usage
|
Description |
β-Pseudouridine is the C-5 glycoside isomer of the nucleoside uridine . It is formed when uridine in RNA undergoes site-specific isomerization by a pseudouridine synthase enzyme. β-pseudouridine is found in tRNAs from bacteria, archaea, and eukaryotes. In vitro, it reduces the number of X-ray-induced chromosomal aberrations in human lymphocytes isolated from whole blood in a dose-dependent manner. |
|
Uses |
An isomer of the nucleoside uridine found in all species and in many classes of RNA except mRNA. It is formed by enzymes called Ψ synthases, which post-transcriptionally isomerize specific uridine residues in RNA in a process termed pseudouridylation. Studies suggest that β-Pseudouridine reduces radiation-induced chromosome aberrations in human lymphocytes. |
|
Definition |
ChEBI: A C-glycosyl pyrimidine that consists of uracil having a beta-D-ribofuranosyl residue attached at position 5. The C-glycosyl isomer of the nucleoside uridine. |
InChI:InChI=1/C9H12N2O6/c12-2-4-5(13)6(14)7(17-4)3-1-10-9(16)11-8(3)15/h1,4-7,12-14H,2H2,(H2,10,11,15,16)/t4-,5-,6-,7+/m1/s1
1445-07-4 Relevant articles
Total synthesis of pseudouridine: Via Heck-type C-glycosylation
Yu, Cheng-Ping,Chang, Hsin-Yun,Chien, Tun-Cheng
, p. 8796 - 8803 (2019)
The reaction of 2,4-dimethoxy-5-iodopyri...
Semi-enzymatic synthesis of pseudouridine
Clerc, Elliot P.,Riley, Andrew T.,Sanford, Tristan C.,Sumita, Minako,Woodard, Austin M.
, (2021)
Modifications of RNA molecules have a si...
-
Brown et al.
, p. 1051 (1968)
-
Structural elucidation of bisulfite adducts to pseudouridine that result in deletion signatures during reverse transcription of RNA
Fleming, Aaron M.,Alenko, Anton,Kitt, Jay P.,Orendt, Anita M.,Flynn, Peter F.,Harris, Joel M.,Burrows, Cynthia J.
, p. 16450 - 16460 (2019)
The recent report of RBS-Seq to map simu...
An arginine-aspartate network in the active site of bacterial TruB is critical for catalyzing pseudouridine formation
Friedt, Jenna,Leavens, Fern M. V.,Mercier, Evan,Wieden, Hans-Joachim,Kothe, Ute
, p. 3857 - 3870 (2014)
Pseudouridine synthases introduce the mo...
-
Asbun,W.,Binkley,S.B.
, p. 140 - 142 (1968)
-
Total Synthesis of Pseudouridimycin
Jia, Yue-Mei,Li, Yi-Xian,Wang, Xu-Kun,Yu, Chu-Yi
, p. 511 - 515 (2022/01/28)
Pseudouridimycin (1), a potent antibioti...
1445-07-4 Process route
-
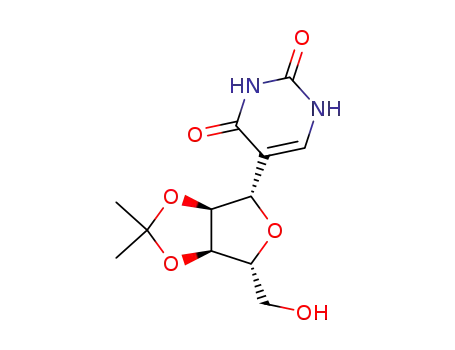
- 28113-58-8
5-(2,3-O-isopropylidene-β-D-ribofuranosyl)uracil

-
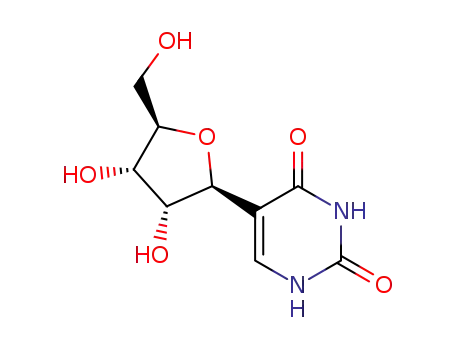
- 1445-07-4
pseudouridine
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; at 20 ℃; for 5h;
|
93% |
-
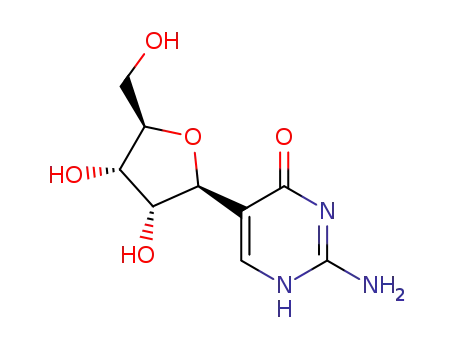
- 57100-18-2
pseudoisocytidine

-

- 1445-07-4
pseudouridine
| Conditions | Yield |
|---|---|
|
With acetic anhydride; In pyridine; ethyl acetate;
|
3.2 g (85%) |
1445-07-4 Upstream products
-
64714-46-1
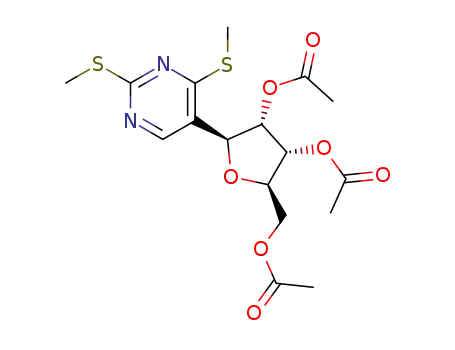
(1S)-tri-O-acetyl-1-(2,4-bis-methylsulfanyl-pyrimidin-5-yl)-D-1,4-anhydro-ribitol
-
67320-14-3
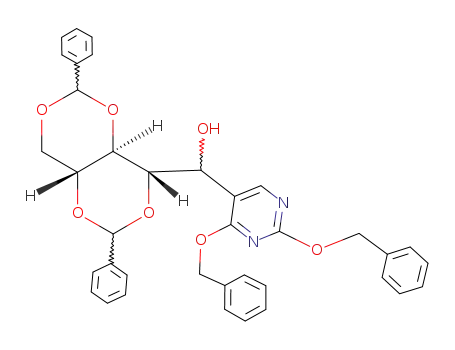
(1Ξ)-O2,O4;O3,O5-(R,R)-dibenzylidene-1-(2,4-bis-benzyloxy-pyrimidin-5-yl)-D-ribitol
-
28113-58-8

5-(2,3-O-isopropylidene-β-D-ribofuranosyl)uracil
-
78119-28-5
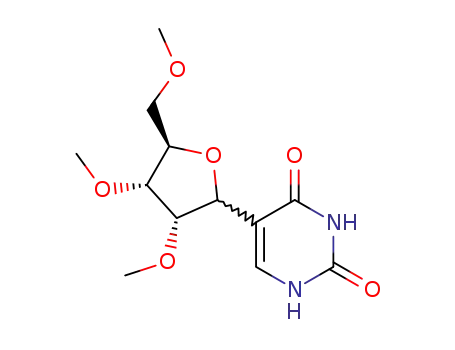
5-((3S,4R,5R)-3,4-Dimethoxy-5-methoxymethyl-tetrahydro-furan-2-yl)-1H-pyrimidine-2,4-dione
1445-07-4 Downstream products
-
13860-38-3
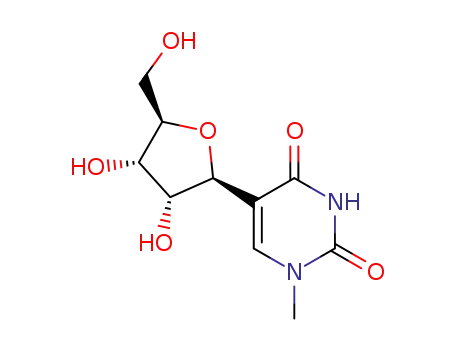
N1-methylpseudouridine
-
65236-78-4
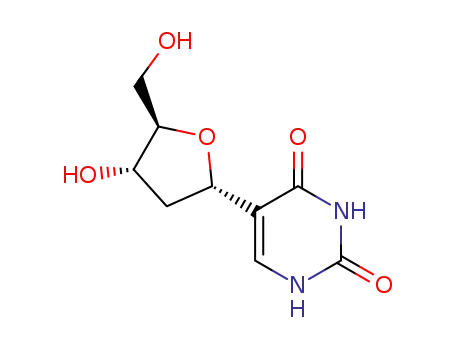
5-(α-D-erythro-2-deoxy-pentofuranosyl)-1H-pyrimidine-2,4-dione
-
39967-60-7
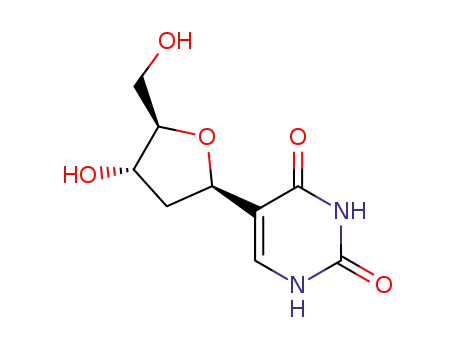
2'-Deoxypseudouridine
-
69265-05-0
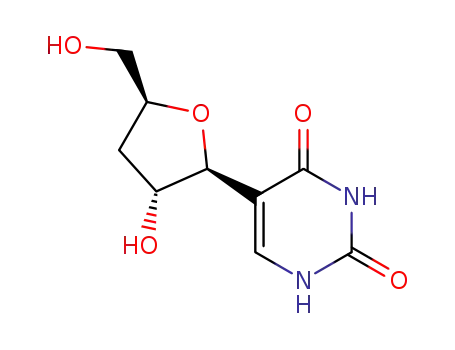
3'-Deoxy-ψ-uridine
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
5-Aminosalicylic acid
CAS:89-57-6
-
5-methyl-3H-1,3,4-thiadiazole-2-thione
CAS:29490-19-5