537-55-3
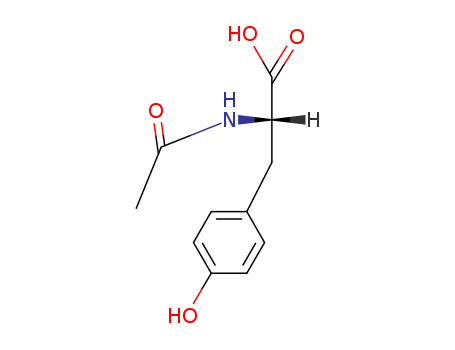
- Product Name:N-acetyl-L-tyrosine
- Molecular Formula:C11H13NO4
- Purity:99%
- Molecular Weight:223.229
Product Details;
CasNo: 537-55-3
Molecular Formula: C11H13NO4
Appearance: solid
Top Purity Factory Supply High Purity N-acetyl-L-tyrosine 537-55-3 with Safe Transportation
- Molecular Formula:C11H13NO4
- Molecular Weight:223.229
- Appearance/Colour:solid
- Vapor Pressure:4.07E-12mmHg at 25°C
- Melting Point:149-152 °C(lit.)
- Refractive Index:1.4960 (estimate)
- Boiling Point:531.3 °C at 760 mmHg
- PKA:3.15±0.10(Predicted)
- Flash Point:275.1 °C
- PSA:86.63000
- Density:1.304 g/cm3
- LogP:0.91490
N-Acetyl-L-tyrosine(Cas 537-55-3) Usage
|
Chemical Properties |
N-Acetyl-L-Tyrosine is a white crystalline powder that is an acetyl derivative of the amino acid, L-tyrosine. It is freely soluble in water compared to L-tyrosine and is commonly used as a parenteral nutrition supplement. N-Acetyl-L-tyrosine , a stable and more soluble form of tyrosine , has been incorporated in some currently available commercial parenteral amino acid formulations such as TrophAmine and Aminosyn-II. |
|
Uses |
N-Acetyl-L-tyrosine is involved in catecholamine production. It can be used as a cell culture media component in the commercial biomanufacture of therapeutic recombinant proteins and monoclonal antibodies. |
|
Definition |
ChEBI: N-acetyl-L-tyrosine is an N-acetyltyrosine in which the chiral centre has L configuration. It has a role as an EC 2.1.1.4 (acetylserotonin O-methyltransferase) inhibitor, a biomarker and a human urinary metabolite. It is a N-acyl-L-tyrosine and a N-acetyltyrosine. It is a conjugate acid of a N-acetyl-L-tyrosinate. |
|
Application |
N-Acetyl-L-tyrosine is a tyrosine derivative with a chemical structure similar to that of an amino acid. It is used as a model system in biochemistry and molecular biology to study the transfer reactions of tyrosine, which are important for energy metabolism, protein synthesis, and metal chelation. N-acetyl-L-tyrosine may be used as an indicator to distinguish neurosyphilis patients from syphilis/non-neurosyphilis patients. N-acetyl-L-tyrosine is a precursor of the essential neurotransmitter dopamine. |
|
benefits |
N-Acetyl-L-Tyrosine (NALT) plays a necessary part in the synthesis of dopamine and other hormones in your body. It can boost levels of the neurotransmitters dopamine, norepinephrine and epinephrine. It helps to support higher levels of focus and cognitive function. It can increase working memory and feelings of well-being. |
|
Side effects |
L-tyrosine gets the generally regarded as safe (GRAS) stamp of approval from the FDA. Some side effects of N-Acetyl-L-tyrosine (NALT) include nausea, headache, fatigue, and heartburn. It can be safely taken for extended periods of time. |
InChI:InChI=1/C11H13NO4/c1-7(13)12-10(11(15)16)6-8-2-4-9(14)5-3-8/h2-5,10,14H,6H2,1H3,(H,12,13)(H,15,16)/p-1/t10-/m0/s1
537-55-3 Relevant articles
Unusual Enhancement of Protease Activity in Organic Solvents by Amines
Yamamoto, Yasuhito,Kise, Hideo
, p. 1821 - 1824 (1993)
The catalytic activities of subtilisin B...
Fluorescence Spectroscopic Study of α-Chymotrypsin as Relevant to Catalytic Activity in Aqueous-Organic Media
Tomiuchi, Yoshimasa,Kijima, Tatsurou,Kise, Hideo
, p. 1176 - 1181 (1993)
The effects of the composition of aqueou...
Effect of dioxane on the binding of competitive inhibitor proflavin and catalytic activity of bovine pancreatic α-chymotrypsin
Sirotkin,Mukhametzyanov,Karmanova
, p. 1160 - 1164 (2007)
The binding of competitive inhibitor pro...
Structure-activity relationship studies of dipeptide-based hepsin inhibitors with Arg bioisosteres
Kwon, Hongmok,Ha, Hyunsoo,Jeon, Hayoung,Jang, Jaebong,Son, Sang-Hyun,Lee, Kiho,Park, Song-Kyu,Byun, Youngjoo
supporting information, (2020/12/25)
Hepsin is a type II transmembrane serine...
GRANZYME B DIRECTED IMAGING AND THERAPY
-
Page/Page column 82; 98, (2019/09/04)
Provided herein are heterocyclic compoun...
Photoinduced electron transfer-promoted debenzylation of phenylalanine and tyrosine derivatives using dicyanoarene
Yamawaki, Mugen,Okita, Yoshiki,Yamamoto, Takashi,Morita, Toshio,Yoshimi, Yasuharu
, p. 7239 - 7244 (2017/11/20)
Photoinduced debenzylations of phenylala...
Radical arylation of tyrosine residues in peptides
Fehler, Stefanie K.,Pratsch, Gerald,?streicher, Christiane,Fürst, Michael C.D.,Pischetsrieder, Monika,Heinrich, Markus R.
supporting information, p. 7888 - 7893 (2016/11/17)
The radical arylation of the phenolic si...
537-55-3 Process route
-
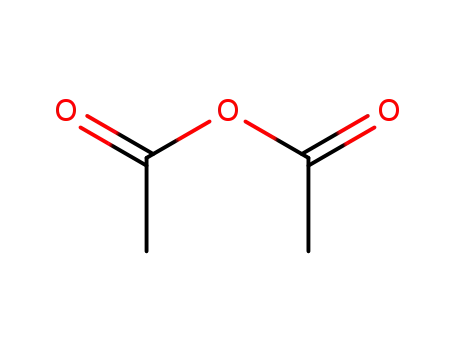
-
108-24-7
acetic anhydride

-
-
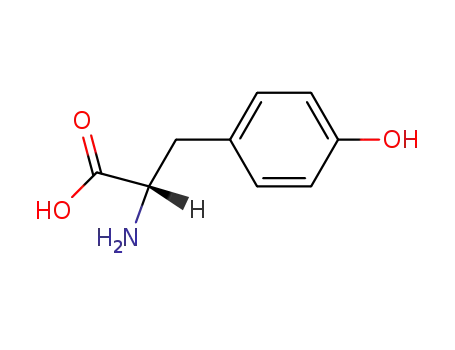
60-18-4,18875-48-4,25619-78-7,30704-25-7
L-tyrosine

-
-
537-55-3
N-acetyl-L-tyrosine
| Conditions | Yield |
|---|---|
|
In
water;
for 4 - 5h;
Heating / reflux;
|
71% |
|
In
water;
for 4 - 5h;
Heating / reflux;
|
71% |
|
With
sodium hydroxide;
|
|
|
With
water;
|
|
|
In
water;
|
|
|
In
water;
at 90 - 100 ℃;
for 2h;
|
|
|
In
water;
at 90 - 95 ℃;
for 2h;
|
|
|
With
sodium hydrogencarbonate;
In
water;
at 0 - 20 ℃;
for 2h;
|
|
|
In
methanol;
at 40 ℃;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 - 20 ℃;
|
|
|
With
sodium hydroxide;
In
water;
at 20 ℃;
pH=14;
|
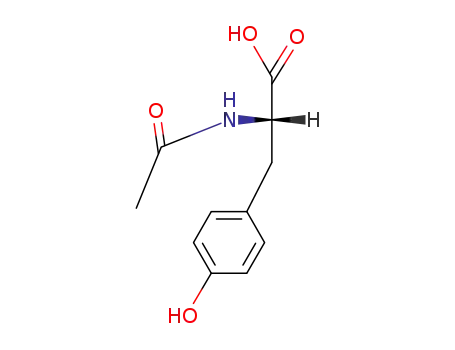
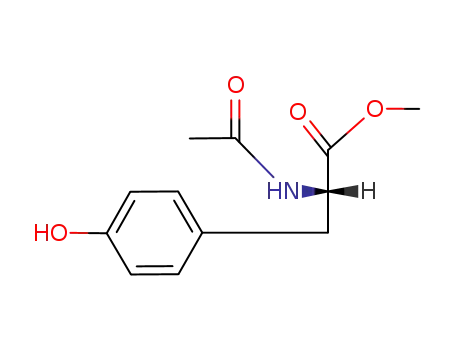
-
-
2440-79-1
N-acetyl-L-tyrosine methyl ester

-

-
537-55-3
N-acetyl-L-tyrosine
| Conditions | Yield |
|---|---|
|
With
lithium hydroxide;
In
tetrahydrofuran; water;
at 65 ℃;
for 3h;
|
84% |
|
With
sodium hydroxide; ethanol;
|
|
|
N-acetyl-L-tyrosine methyl ester;
With
lithium hydroxide; water;
In
tetrahydrofuran;
at 60 ℃;
for 1h;
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 ℃;
pH=4;
Reactivity;
|
|
|
N-acetyl-L-tyrosine methyl ester;
With
lithium hydroxide; water;
In
tetrahydrofuran;
at 60 ℃;
for 1h;
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 ℃;
pH=4;
|
537-55-3 Upstream products
-
840-97-1
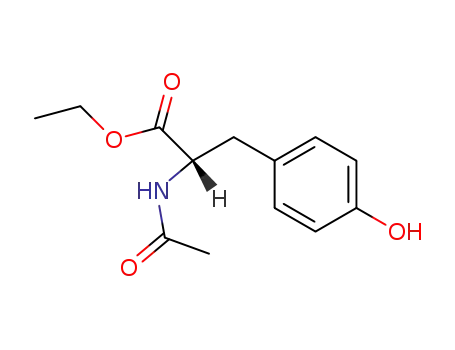
N-acetyl-L-tyrosine ethyl ester
-
2440-79-1

N-acetyl-L-tyrosine methyl ester
-
949-67-7
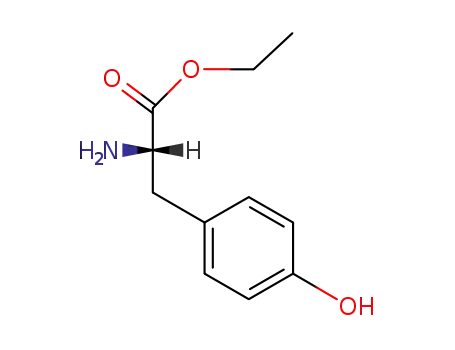
L-tyrosine ethyl ester
-
108-24-7

acetic anhydride
537-55-3 Downstream products
-
17355-24-7
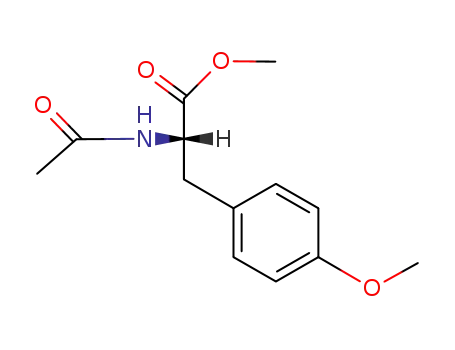
(S)-N-acetyl-3-(4-methoxyphenyl)alanine methyl ester
-
2440-79-1

N-acetyl-L-tyrosine methyl ester
-
840-97-1

N-acetyl-L-tyrosine ethyl ester
-
20767-00-4
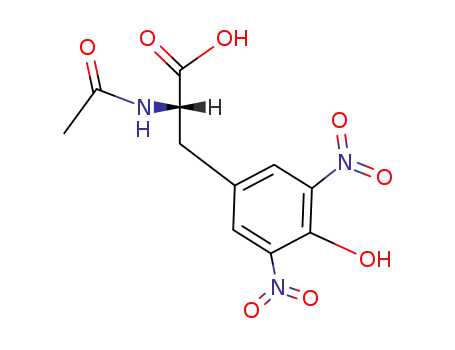
3.5-Dinitro-N-acetyl-L-tyrosin
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
Uridine
CAS:58-96-8
-
5-Aminosalicylic acid
CAS:89-57-6