59278-00-1
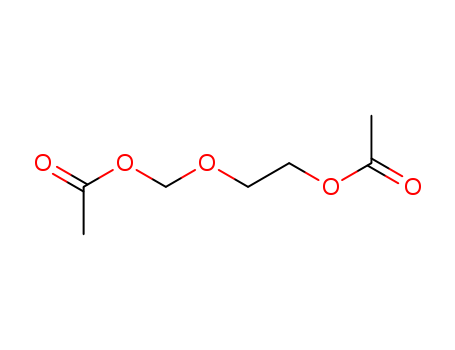
- Product Name:2-[(Acetyloxy)methoxy]ethyl acetate
- Molecular Formula:C7H12O5
- Purity:99%
- Molecular Weight:176.169
Product Details;
CasNo: 59278-00-1
Molecular Formula: C7H12O5
Appearance: clear, colourless or yellowish liquid
Factory Sells Reputable Manufacturer Supply 2-[(Acetyloxy)methoxy]ethyl acetate 59278-00-1 On Stock
- Molecular Formula:C7H12O5
- Molecular Weight:176.169
- Appearance/Colour:clear, colourless or yellowish liquid
- Vapor Pressure:0.0874mmHg at 25°C
- Refractive Index:1.419
- Boiling Point:225.2 °C at 760 mmHg
- Flash Point:90.9 °C
- PSA:61.83000
- Density:1.119 g/cm3
- LogP:0.08670
2-[(Acetyloxy)methoxy]ethyl acetate(Cas 59278-00-1) Usage
|
Chemical Properties |
Bright Yellow Liquid |
|
Uses |
Intermediate for the preparation of Acyclovir-d4 |
InChI:InChI=1/C7H12O5/c1-6(8)11-4-3-10-5-12-7(2)9/h3-5H2,1-2H3
59278-00-1 Relevant articles
Synthesis and antitumor activity of an acyclonucleoside derivative of 5-fluorouracil
Rosowsky,Kim,Wick
, p. 1177 - 1181 (1981)
-
Acyclovir preparation device and preparation method
-
Page/Page column 7-11, (2019/10/04)
The invention discloses an acyclovir pre...
Diverse combinatorial design, synthesis and in vitro evaluation of new HEPT analogues as potential non-nucleoside HIV-1 reverse transcription inhibitors
Puig-De-La-Bellacasa, Raimon,Gimenez, Laura,Pettersson, Sofia,Pascual, Rosalia,Gonzalo, Encarna,Este, Jose A.,Clotet, Bonaventura,Borrell, Jose I.,Teixido, Jordi
scheme or table, p. 159 - 174 (2012/09/05)
New analogues of 1-[(2-hydroxyethoxy)met...
Novel 5-(N -alkylaminouracil) acyclic nucleosides
Boncel, Sawomir,Gondela, Andrzej,McZka, MacIej,Tuszkiewicz-Kuznik, Magdalena,Grec, Przemysaw,Hefczyc, Barbara,Walczak, Krzysztof
experimental part, p. 603 - 610 (2011/04/12)
Protocols for the two-step syntheses of ...
Synthesis of oligoribonucleotides containing pyrimidine 2′-O-[(hydroxyalkoxy)methyl]ribonucleosides
Bobkov, Georgii V.,Brilliantov, Kirill V.,Mikhailov, Sergey N.,Rozenski, Jef,Van Aerschot, Arthur,Herdewijn, Piet
, p. 804 - 819 (2008/02/02)
A simple and efficient method for the pr...
59278-00-1 Process route
-
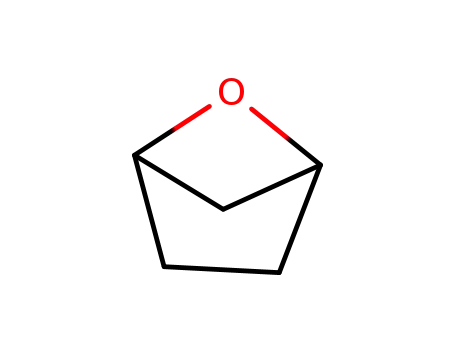
-
1,3-epoxycyclopentane

-
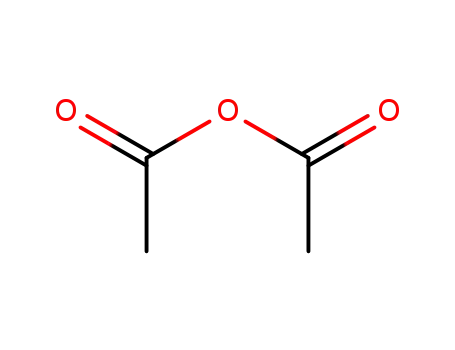
-
108-24-7
acetic anhydride

-
-
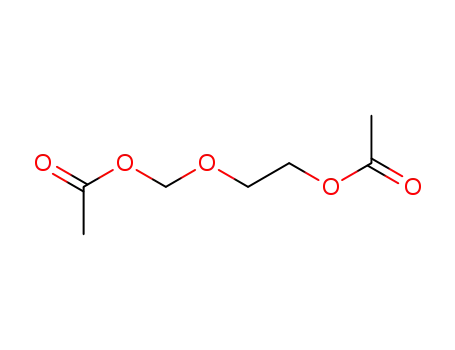
59278-00-1
2-acetoxyethyl acetoxymethyl ether
| Conditions | Yield |
|---|---|
|
With
toluene-4-sulfonic acid;
at 35 - 40 ℃;
for 0.833333h;
Reagent/catalyst;
Temperature;
|
91% |
-
-
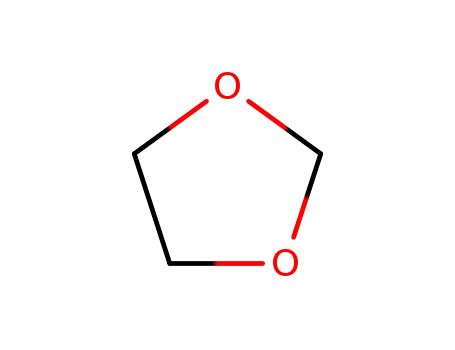
646-06-0
1,3-DIOXOLANE

-

-
108-24-7
acetic anhydride

-

-
59278-00-1
2-acetoxyethyl acetoxymethyl ether
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
Ambient temperature;
|
74% |
|
With
sulfuric acid;
at -5 - 20 ℃;
|
52% |
|
With
iron(III) chloride;
|
|
|
|
|
|
With
sulfuric acid; acetic acid;
|
|
|
With
sulfuric acid;
|
59278-00-1 Upstream products
-
646-06-0

1,3-DIOXOLANE
-
108-24-7

acetic anhydride
-
506-96-7
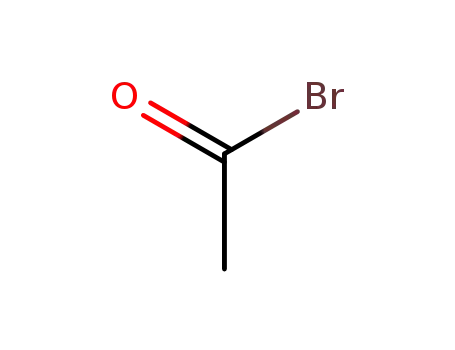
Acetyl bromide
-
7664-93-9
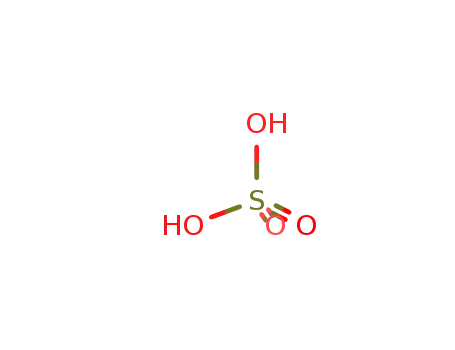
sulfuric acid
59278-00-1 Downstream products
-
77474-49-8
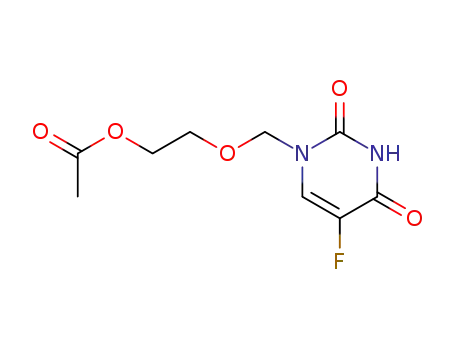
1-<(2-acetoxyethoxy)methyl>-5-fluorouracil
-
75128-73-3
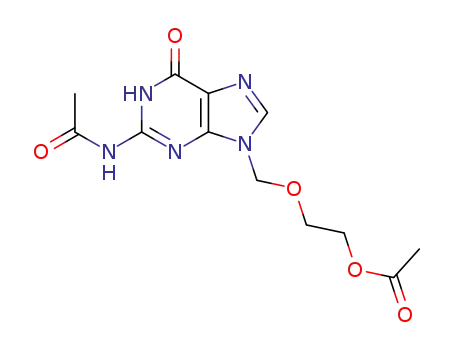
2-acetylamino-9-(2-acetoxyethoxymethyl)purine-6-one
-
91702-60-2
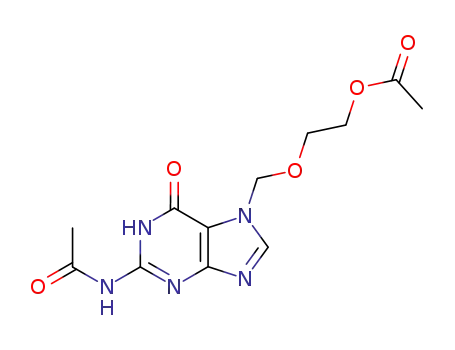
7-<(2-acetoxyethoxy)methyl>-N2-acetylguanine
-
102728-64-3
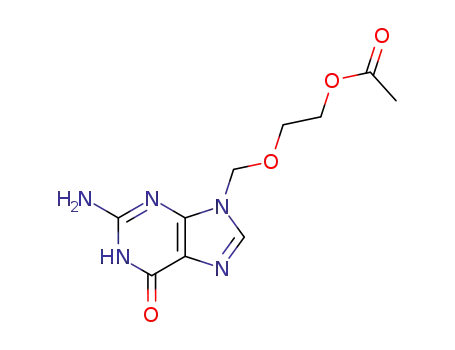
9-[(acetoxyethoxy)methyl]guanine
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
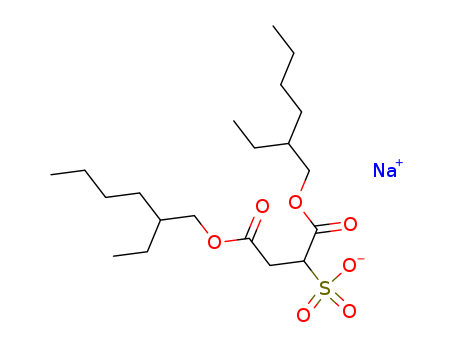
Dioctyl sulfosuccinate, sodium salt
CAS:577-11-7
-
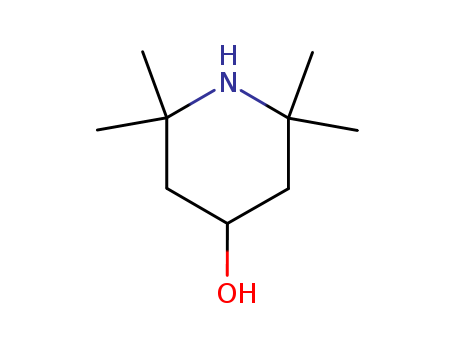
2,2,6,6-Tetramethyl-4-piperidinol
CAS:2403-88-5