577-11-7
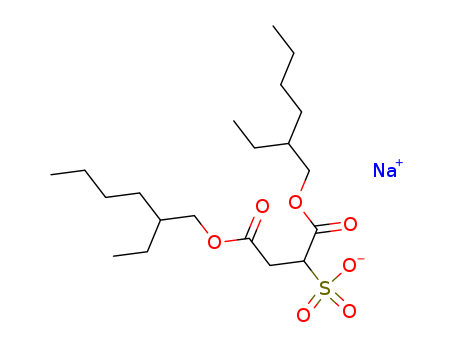
- Product Name:Dioctyl sulfosuccinate, sodium salt
- Molecular Formula:C20H37NaO7S
- Purity:99%
- Molecular Weight:444.565
Product Details;
CasNo: 577-11-7
Molecular Formula: C20H37NaO7S
Appearance: odorless colorless to white waxy solid
Reliable Quality Reputable Manufacturer Supply Dioctyl sulfosuccinate, sodium salt 577-11-7 with Safe Transportation
- Molecular Formula:C20H37NaO7S
- Molecular Weight:444.565
- Appearance/Colour:odorless colorless to white waxy solid
- Vapor Pressure:0Pa at 25℃
- Melting Point:153-157 °C
- Boiling Point:82.7°C
- Flash Point:91-95 °C
- PSA:118.18000
- Density:1.1 g/cm3
- LogP:4.89030
Docusate sodium(Cas 577-11-7) Usage
|
Chemical Properties |
white solid, often supplied as an aqueous solution |
|
Uses |
dioctyl sodium sulfosuccinate is a mild surfactant used as a cleans ing agent. |
|
Production Methods |
Maleic anhydride is treated with 2-ethylhexanol to produce dioctyl maleate, which is then reacted with sodium bisulfite. |
|
Brand name |
Colace (Roberts Pharmaceutical); Correctol Stool Softener Laxative (Schering-Plough HealthCare); Dialose (Johnson & Johnson-Merck Consumer); Doxinate (Hoechst-Roussel); D-S-S (Parke-Davis); Modane Soft (Savage); Molofac (Bristol-Myers Squibb). |
|
General Description |
Odorless colorless to white waxy solid. Sinks and mixes slowly with water. |
|
Air & Water Reactions |
Mixes slowly with water. |
|
Reactivity Profile |
Docusate sodium causes foaming and spreading of water. Assists in putting out fires by water. [USCG, 1999]. |
|
Health Hazard |
Liquid is strong irritant to eye and may irritate skin by removing natural oils. Ingestion causes diarrhea and intestinal bloating. |
|
Fire Hazard |
Behavior in Fire: Causes foaming and spreading of water. Assists in putting out fires by water. |
|
Flammability and Explosibility |
Nonflammable |
|
Pharmaceutical Applications |
Docusate sodium and docusate salts are widely used as anionic surfactants in pharmaceutical formulations. Docusate sodium is mainly used in capsule and direct-compression tablet formulations to assist in wetting and dissolution. |
|
Safety Profile |
Poison by intravenous route. Moderately toxic by ingestion and intraperitoneal routes. A skin and severe eye irritant. See also ESTERS. When heated to decomposition it emits toxic fumes of SOx and Na2O. |
|
Safety |
Docusate salts are used in oral formulations as therapeutic agents for their fecal softening and laxative properties. As a laxative in adults, up to 500mg of docusate sodium is administered daily in divided doses; in children over 6 months old, up to 75 mg in divided doses is used. The quantity of docusate sodium used as an excipient in oral formulations should therefore be controlled to avoid unintended laxative effects. Adverse effects associated with docusate sodium include diarrhea, nausea, vomiting, abdominal cramps, and skin rashes. As with the chronic use of laxatives, the excessive use of docusate sodium may produce hypomagnesemia. Docusate salts are absorbed from the gastrointestinal tract and excreted in bile; they may cause alteration of the gastrointestinal epithelium. The gastrointestinal or hepatic absorption of other drugs may also be affected by docusate salts, enhancing activity and possibly toxicity. Docusate sodium should not be administered with mineral oil as it may increase the absorption of the oil. LD50 (mouse, IV): 0.06 g/kg LD50 (mouse, oral): 2.64 g/kg LD50 (rat, IP): 0.59 g/kg LD50 (rat, oral): 1.9 g/kg |
|
Solubility in organics |
Dioctyl sodium sulfosuccinate (DSS) is the dioctyl ester of sodium sulfosuccinate (bis-2-ethyl-hexyl sodium sulfosuccinate). It dissolves slowly in water; at 25°C to the extent of 1.5 gm/100cc; at 70°C, 5.5 gm/100cc. It dissolves in oils, hydrocarbons, fats and waxs by heating above 75°C and remains in solution when cooled to room temperature. At room temperature, it is readily soluble in most organic solvents, both polar and non-polar. soluble in carbon tetrachloride, petroleum ether, naphtha, xylene, dibutyl phthalate, liquid petroleum, acetone, alcohol, vegetable oils. |
|
storage |
Docusate sodium is stable in the solid state when stored at room temperature. Dilute aqueous solutions of docusate sodium between pH 1–10 are stable at room temperature. However, at very low pH (<1) and very high pH (>10) docusate sodium solutions are subject to hydrolysis. The solid material is hygroscopic and should be stored in an airtight container in a cool, dry place. |
|
Purification Methods |
Dissolve it in MeOH and the inorganic salts which precipitate are filtered off. Water is added and the solution is extracted several times with hexane. The residue is evaporated to one-fifth its original volume, *benzene is added and azeotropic distillation is continued until no water remains. The solvent is evaporated. The white residual solid is crushed and dried in vacuo over P2O5 for 48hours [El Seoud & Fendler J Chem Soc, Faraday Trans 1 71 452 1975]. [Beilstein 4 IV 114.] It solubilises major myelin trans membrane proteolipids, and forms reverse micelles in hydrocarbon solvents. |
|
Incompatibilities |
Electrolytes, e.g. 3% sodium chloride, added to aqueous solutions of docusate sodium can cause turbidity. However, docusate sodium possesses greater tolerance to calcium, magnesium, and other polyvalent ions than do some other surfactants. Docusate sodium is incompatible with acids at pH < 1 and with alkalis at pH > 10. |
|
Regulatory Status |
GRAS listed. Included in the FDA Inactive Ingredients Database (IM injections; oral capsules, suspensions, and tablets; also topical formulations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Who Evaluation |
Evaluation year: 1995 |
InChI:InChI=1/C20H38O7S.Na/c1-5-9-11-16(7-3)14-26-19(21)13-18(28(23,24)25)20(22)27-15-17(8-4)12-10-6-2;/h16-18H,5-15H2,1-4H3,(H,23,24,25);/q;+1
577-11-7 Relevant articles
Dual catalytic activity of amberlyst-15 in the large-scale and sustainable synthesis of dioctyl sodium sulfosuccinate (DOSS)
Fateh, Davod S.,Moghadam, Seyed M. M.,Salehi, Samie,Tarin, Mojtaba
, p. 555 - 559 (2020/07/17)
Dioctyl sodium sulfosuccinate (DOSS) as ...
Method for producing maleic acid disooctyl sodium sulfonate
-
Paragraph 0019-0028, (2019/12/25)
The invention provides a method for prod...
Salts of alkyl esters of sulfonated dicarboxylic acids and compositions containing same
-
Page/Page column 4, (2011/08/03)
A salt of a mono- and/or dialkyl ester o...
Microstructure and magnetic properties of colloidal cobalt nano-clusters
Torchio,Meneghini,Mobilio,Capellini,Garcia Prieto,Alonso,Fdez-Gubieda,Turco Liveri,Longo,Ruggirello,Neisius
scheme or table, p. 3565 - 3571 (2011/01/10)
The magnetic response of nanometer sized...
577-11-7 Process route
-
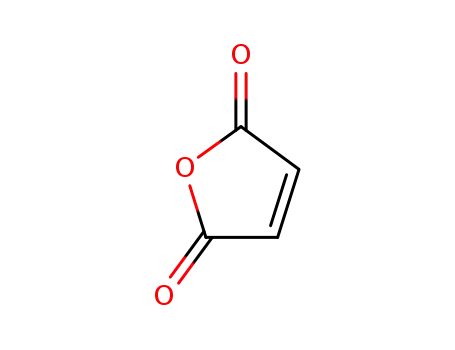
-
108-31-6
maleic anhydride

-
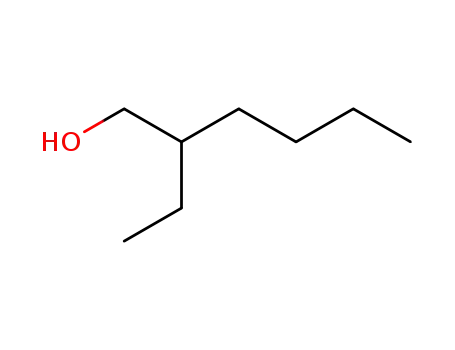
-
104-76-7
2-Ethylhexyl alcohol

-
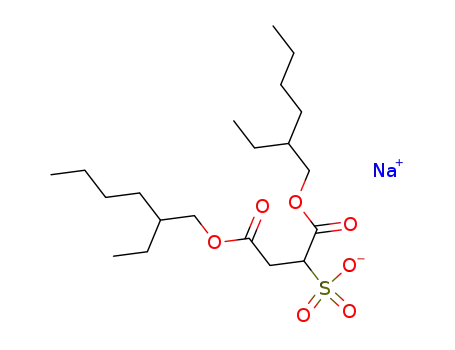
-
577-11-7
sodium docusate
| Conditions | Yield |
|---|---|
|
maleic anhydride; 2-Ethylhexyl alcohol;
at 60 - 130 ℃;
for 3.5h;
under 760.051 Torr;
With
sodium hydrogensulfite; sodium hydroxide;
In
water;
at 107 ℃;
for 1h;
Reagent/catalyst;
Temperature;
|
98.2% |
-
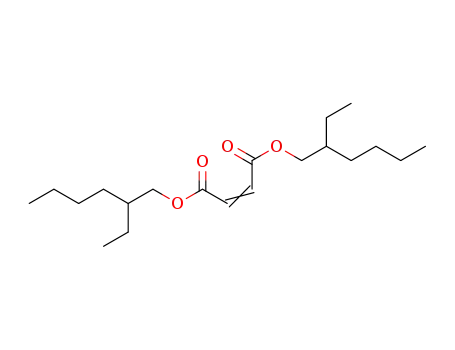
-
128111-61-5,77666-69-4
bis(2-ethylhexyl) maleate

-

-
577-11-7
sodium docusate
| Conditions | Yield |
|---|---|
|
With
sodium metabisulfite;
In
ethanol;
for 10h;
Temperature;
Time;
Inert atmosphere;
Reflux;
|
94% |
577-11-7 Upstream products
-
142-16-5
bis(2-ethylhexyl) maleate
-
108-31-6

maleic anhydride
-
104-76-7

2-Ethylhexyl alcohol
-
128111-61-5

bis(2-ethylhexyl) maleate
577-11-7 Downstream products
-
10041-19-7
bis(2-ethylhexyl) sulfosuccinate
-
104-76-7

2-Ethylhexyl alcohol
-
24273-80-1
4-n-hexadecyl-2,6-dimethylphenol
-
114-07-8
erythromycin A
Relevant Products
-
Sodium risedronate
CAS:115436-72-1
-
Entecavir
CAS:142217-69-4
-
2-[(Acetyloxy)methoxy]ethyl acetate
CAS:59278-00-1