2403-88-5
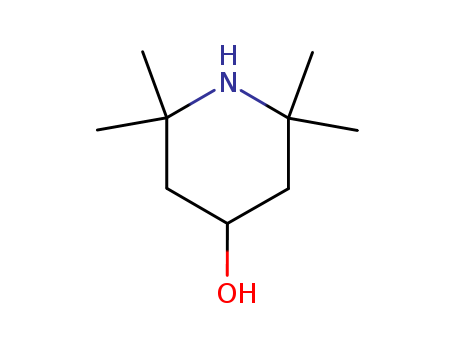
- Product Name:2,2,6,6-Tetramethyl-4-piperidinol
- Molecular Formula:C9H19NO
- Purity:99%
- Molecular Weight:157.256
Product Details;
CasNo: 2403-88-5
Molecular Formula: C9H19NO
Appearance: white to slightly beige crystalline powder
Buy Quality Factory Supply 2,2,6,6-Tetramethyl-4-piperidinol 2403-88-5 with Cheap Price
- Molecular Formula:C9H19NO
- Molecular Weight:157.256
- Appearance/Colour:white to slightly beige crystalline powder
- Vapor Pressure:0.035mmHg at 25°C
- Melting Point:129-131 °C(lit.)
- Refractive Index:1.4248 (estimate)
- Boiling Point:214.1 °C at 760 mmHg
- PKA:14.99±0.60(Predicted)
- Flash Point:53 °C
- PSA:32.26000
- Density:0.891 g/cm3
- LogP:1.61670
2,2,6,6-Tetramethyl-4-piperidinol(Cas 2403-88-5) Usage
|
Chemical Properties |
white to slightly beige crystalline powder |
|
Uses |
An intermediate in the preparation of Piperidinyloxy free radical derivatives. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 37, p. 2050, 1972 DOI: 10.1021/jo00977a047 |
|
Purification Methods |
The piperidine crystallises from water as a hydrate and crystallises from dry ether or *C6H6 as the anhydrous base. The hydrochloride has m 282-284o (from EtOH/H2O), and the formate has m 207o(dec, from EtOH/EtOAc). [Mailey & Day J Org Chem 22 1061 1957, Beilstein 21 I 195, 21 III/IV 146, 21/1 V 159.] |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C9H19NO/c1-8(2)5-7(11)6-9(3,4)10-8/h7,10-11H,5-6H2,1-4H3/p+1
2403-88-5 Relevant articles
-
Chen,Le Fevre
, p. 3467,3470 (1965)
-
-
Kornblum,Pinnick
, p. 2050 (1972)
-
ELECTROCHEMICAL SYNTHESIS OF 2,2,6,6,-TETRAMETHYLPIPERIDINE
Kagan E. Sh.,Avrutskaya, I. A.,Kondrashov, S. V.,Novikov, V. T.,Fioshin, M. Ya.,Smirnov, V. A.
, p. 288 - 289 (1984)
A preparative method for the production ...
Reductive amination of triacetoneamine with n-butylamine over Cu-Cr-La/γ-Al2O3
Sun, Meng,Du, Xiaobao,Wang, Huabang,Wu, Zhiwei,Li, Yang,Chen, Ligong
, p. 1703 - 1708 (2011)
A series of Cu-based catalysts were prep...
Crowded piperidines with intramolecularly hydrogen-bonded nitrogen: Synthesis and conformation study
Belostotskii, Anatoly M.,Gottlieb, Hugo E.,Aped, Pinchas
, p. 3016 - 3026 (2002)
2,2,6,6-Tetramethyl substituted piperidi...
-
Bellus et al.
, p. 1199 (1972)
-
A continuous process for the production of 2,2,6,6-tetramethylpiperidin-4- ol catalyzed by Cu-Cr/γ-Al2O3
Fan, Xiaopeng,Liu, Shuai,Yan, Xilong,Du, Xiaobao,Chen, Ligong
, p. 960 - 963 (2010)
A continuous processwas established for ...
Chemistry and anti-herpes simplex virus type 1 evaluation of 4-substituted-1H-1,2,3-triazole-nitroxyl-linked hybrids
Cunha, Anna C.,Ferreira, Vitor F.,Vaz, Maria G. F.,Cassaro, Rafael A. All?o,Resende, Jackson A. L. C.,Sacramento, Carolina Q.,Costa, Jéssica,Abrantes, Juliana L.,Souza, Thiago Moreno L.,Jord?o, Alessandro K.
, p. 2035 - 2043 (2020/05/25)
Abstract: HSV disease is distributed wor...
General methodology for the chemoselective N-alkylation of (2,2,6,6)-tetramethylpiperidin-4-ol: Contribution of microwave irradiation
Membrat, Romain,Vasseur, Alexandre,Giordano, Laurent,Martinez, Alexandre,Nuel, Didier
supporting information, p. 240 - 243 (2019/01/04)
A convenient method to access a broad va...
Phosphinous Acid Platinum Complex as Robust Catalyst for Oxidation: Comparison with Palladium and Mechanistic Investigations
Membrat, Romain,Vasseur, Alexandre,Martinez, Alexandre,Giordano, Laurent,Nuel, Didier
supporting information, p. 5427 - 5434 (2018/10/20)
Secondary phosphine oxides proved to be ...
2′-Alkynylnucleotides: A Sequence- and Spin Label-Flexible Strategy for EPR Spectroscopy in DNA
Haugland, Marius M.,El-Sagheer, Afaf H.,Porter, Rachel J.,Pe?a, Javier,Brown, Tom,Anderson, Edward A.,Lovett, Janet E.
supporting information, p. 9069 - 9072 (2016/08/05)
Electron paramagnetic resonance (EPR) sp...
2403-88-5 Process route
-
-
826-36-8
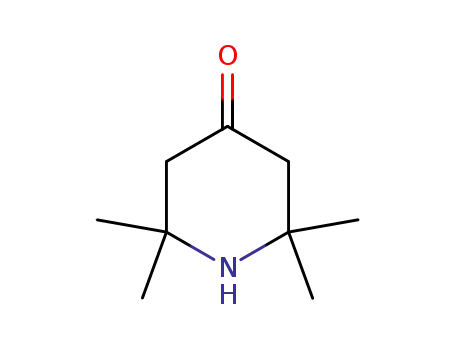
2,2,6,6-Tetramethyl-4-piperidone

-
-
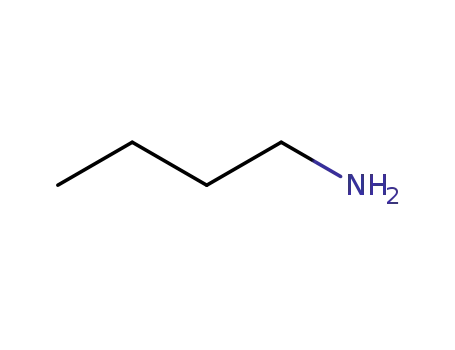
109-73-9,85404-21-3
N-butylamine

-
-
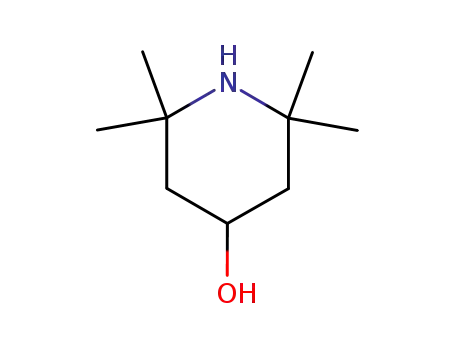
2403-88-5
4-hydroxy-2,2,6,6-tetramethylpiperidine

-
-
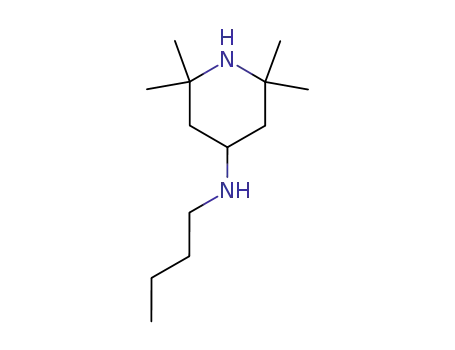
36177-92-1
4-n-butylamino-2,2,6,6-tetramethylpiperidine
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
1,4-dioxane;
at 120 ℃;
under 30003 Torr;
|
-

-
826-36-8
2,2,6,6-Tetramethyl-4-piperidone

-
-
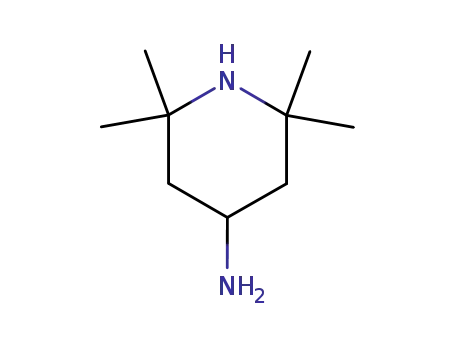
36768-62-4
4-AMINO-2,2,6,6-TETRAMETHYLPIPERIDINE

-

-
2403-88-5
4-hydroxy-2,2,6,6-tetramethylpiperidine
| Conditions | Yield |
|---|---|
|
With
ammonium sulfate;
electrosynthesis, var. pH;
|
|
|
With
ammonia; hydrogen;
B113W;
In
water;
at 55 - 100 ℃;
for 3h;
under 30003 Torr;
Product distribution / selectivity;
|
92.10 %Chromat. 5.49 %Chromat. |
2403-88-5 Upstream products
-
36768-62-4

4-AMINO-2,2,6,6-TETRAMETHYLPIPERIDINE
-
826-36-8

2,2,6,6-Tetramethyl-4-piperidone
-
45985-24-8
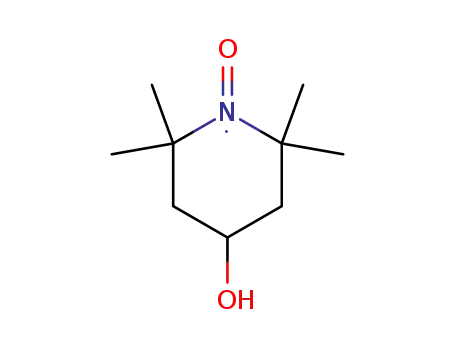
TEMPOL
-
451-40-1
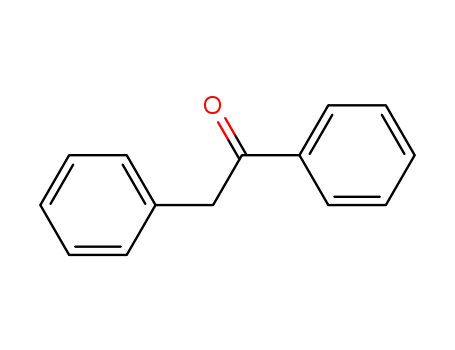
phenyl benzyl ketone
2403-88-5 Downstream products
-
2403-89-6
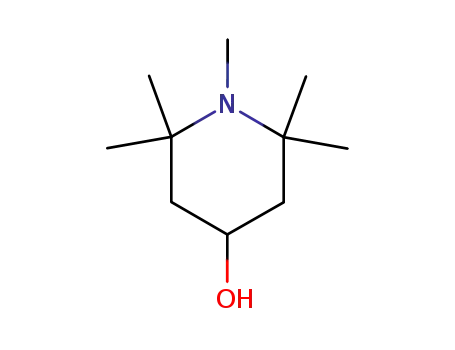
1,2,2,6,6-pentamethyl-piperidin-4-ol
-
26275-88-7
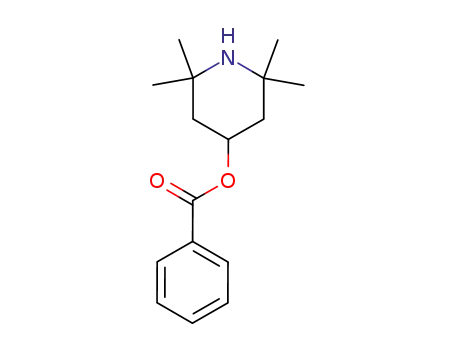
4-benzoyloxy-2,2,6,6-tetramethylpiperidine
-
100444-13-1
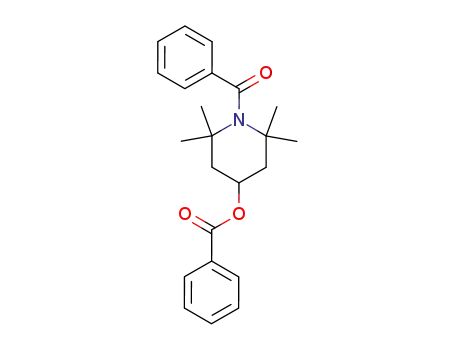
1-benzoyl-4-benzoyloxy-2,2,6,6-tetramethyl-piperidine
-
101866-33-5
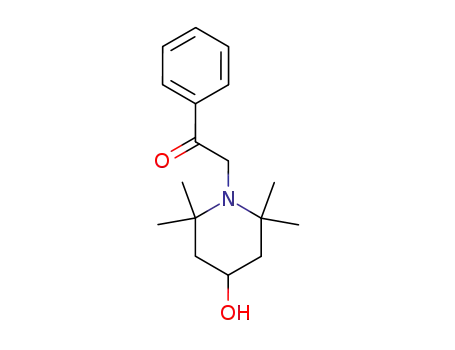
2-(4-hydroxy-2,2,6,6-tetramethyl-piperidino)-1-phenyl-ethanone
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
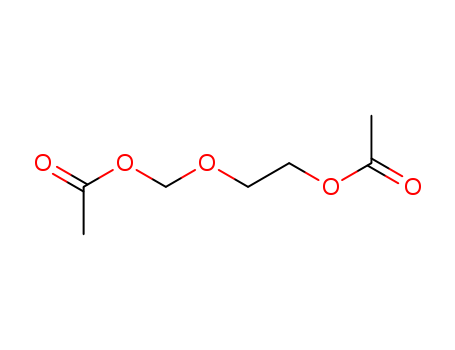
2-[(Acetyloxy)methoxy]ethyl acetate
CAS:59278-00-1
-
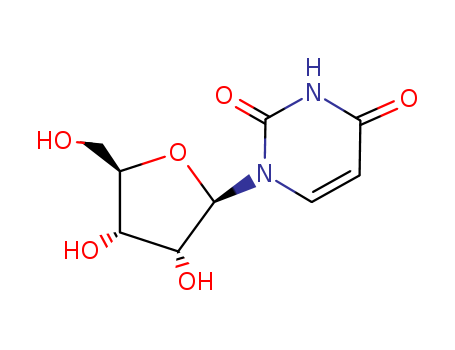
Uridine
CAS:58-96-8