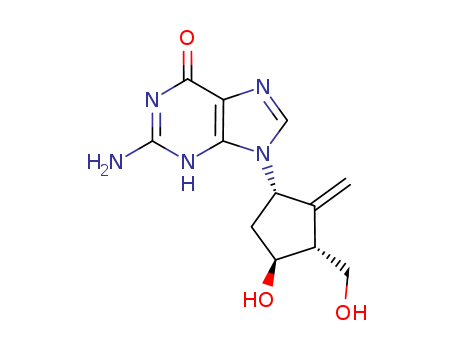
142217-69-4
- Product Name:Entecavir
- Molecular Formula:C12H15N5O3
- Purity:99%
- Molecular Weight:277.283
Product Details;
CasNo: 142217-69-4
Molecular Formula: C12H15N5O3
Appearance: white to off-white/yellow crystalline powder
Best Quality Trustworthy Manufacturer Supply Entecavir 142217-69-4 with Efficient Delivery
- Molecular Formula:C12H15N5O3
- Molecular Weight:277.283
- Appearance/Colour:white to off-white/yellow crystalline powder
- Melting Point:249-252 °C
- Refractive Index:1.837
- Boiling Point:734.2 °C at 760 mmHg
- PKA:14.22±0.60(Predicted)
- Flash Point:397.9 °C
- PSA:130.05000
- Density:1.81 g/cm3
- LogP:-0.24660
Entecavir(Cas 142217-69-4) Usage
|
Description |
Entecavir is a cyclopentyl guanosine analog launched for the once-daily oral treatment of chronic hepatitis B virus (HBV) infection, and it is the third nucleoside or nucleotide analog to be marketed for this indication. Lamivudine, a deoxythiacytosine analog, and adefovir dipivoxil, a nucleotide analog, have been marketed since 1998 and 2002, respectively. Entecavir and adefovir are specifically indicated for HBV, whereas lamivudine is indicated for both HBV and HIV infections. |
|
Chemical Properties |
White to Off-White/Yellow Crystalline Powder |
|
Originator |
BMS (US) |
|
Uses |
Entecavir is a new generation of guanine nucleoside analogues oral medicine for treatment of hepatitis B virus infection in, mainly for the treatment of adult patients with viral replication activity and serum transaminase continued to increase, or liver tissue for pathological activity of chronic hepatitis B, is currently down virus the fastest and the most powerful, the mutation rate lowest nucleoside analogues. |
|
Definition |
ChEBI: Guanine substituted at the 9 position by a 4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl group. A synthetic analogue of 2'-deoxyguanosine, it is a nucleoside reverse transcriptase inhibitor with selective antiviral activity against hepatitis B virus Entecavir is phosphorylated intracellularly to the active triphosphate form, which competes with deoxyguanosine triphosphate, the natural substrate of hepatitis B virus reverse transcriptase, inhibiting every stage of the enzyme's activity, although it ha no activity against HIV. It is used for the treatment of chronic hepatitis B. |
|
Mechanism of action |
Entecavir is a nucleoside analog, or more specifically, a deoxyguanosine analogue that belongs to a class of carbocyclic nucleosides and inhibits reverse transcription, DNA replication and transcription in the viral replication process. |
|
Pharmacokinetics |
Entecavir had a mean terminal half-life ranging from 128 to 149 hours and an effective half-life of approximately 24 hours. Elimination was predominantly through renal excretion, with mean urinary recovery ranging from 62% to 73%. |
|
Clinical Use |
Treatment of chronic hepatitis B virus infection in patients >16 years of age.Entecavir comes as a tablet and solution (liquid) to take by mouth. It is usually taken once a day on an empty stomach, at least 2 hours after a meal and at least 2 hours before the next meal. Take entecavir at around the same time every day. |
|
Side effects |
The most common side effects of entecavir: the increase of ALT, fatigue, dizziness, nausea, abdominal pain, abdominal discomfort, abdominal discomfort, liver, muscle, insomnia, rubella and indigestion, also be found in neutrophils decreased slightly. These adverse reactions were mild to moderate. It also found that, as the same type of antiviral drugs, entecavir and the first generation of antiviral drugs have similar side effects, such as acid poisoning, hepatomegaly, liver fatty degeneration in the withdrawal will appear rebound phenomenon. |
|
Synthesis |
Entecavir is synthesized from 4-trimethylsilyl-3-butyn-2-one and acrolein. The key features of its preparation are: (1) a stereoselective boron–aldol reaction to afford the acyclic carbon skeleton of the methylenecylopentane moiety; (2) its cyclization by a Cp2TiCl-catalyzed intramolecular radical addition of an epoxide to an alkyne; and (3) the coupling with a purine derivative by a Mitsunobu reaction. |
InChI:InChI=1/C12H15N5O3/c1-5-6(3-18)8(19)2-7(5)17-4-14-9-10(17)15-12(13)16-11(9)20/h4,6-8,18-19H,1-3H2,(H3,13,15,16,20)/t6-,7-,8+/m0/s1
142217-69-4 Relevant articles
A novel and efficient synthesis of Entecavir
Liu, Xiaoyu,Jiao, Xiaozhen,Wu, Qian,Tian, Chengsen,Li, Renze,Xie, Ping
, p. 3805 - 3807 (2012)
A practical synthesis of Entecavir (1) h...
Total Synthesis of Entecavir: A Robust Route for Pilot Production
Xu, Hua,Wang, Fang,Xue, Weicai,Zheng, Yunjie,Wang, Qi,Qiu, Fayang G.,Jin, Yehua
, p. 377 - 384 (2018)
A practical synthetic route for pilot pr...
Novel preparation method and intermediates of entecavir
-
, (2021/04/10)
The invention provides novel intermediat...
Improved entecavir intermediate synthesis process and improved entecavir synthesis process
-
, (2020/10/14)
The invention discloses an improved ente...
Entecavir intermediate, synthetic method thereof and synthetic method of entecavir
-
, (2020/07/29)
The invention belongs to the technical f...
Method for synthesizing entecavir
-
, (2019/04/27)
The invention provides a method for synt...
142217-69-4 Process route
-
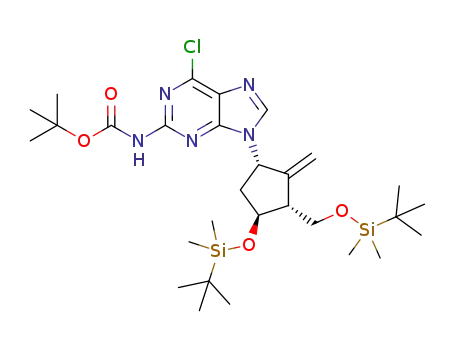
-
1354715-15-3
9-((1S,3R,4S)-4-(tert-butyldimethylsilyloxy)-3-(tertbutyldimethyl-silyloxymethyl)-2-methylenecyclopentyl)-6-chloro-9H-purine-2-carbamic acid tert-butyl ester

-
-
142217-69-4,76704-05-7
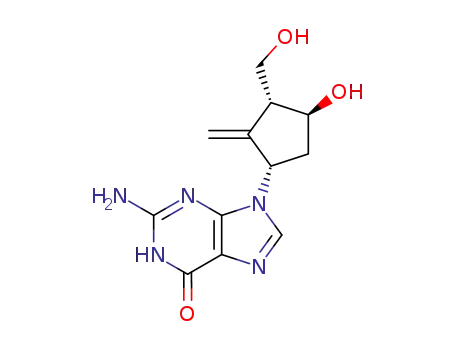
entecavir
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 60 - 65 ℃;
|
90% |
|
With
hydrogenchloride; water;
In
tetrahydrofuran;
Heating;
|
75% |
|
With
hydrogenchloride; water;
In
tetrahydrofuran;
|
75% |
|
With
hydrogenchloride;
In
tetrahydrofuran;
|
75% |
|
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 - 55 ℃;
for 11h;
Inert atmosphere;
|
73% |
|
Multi-step reaction with 2 steps
1: hydrogenchloride / methanol; tetrahydrofuran / 2 h / 20 °C
2: hydrogenchloride; water / tetrahydrofuran / 6 h / Reflux
With
hydrogenchloride; water;
In
tetrahydrofuran; methanol;
|
|
|
Multi-step reaction with 2 steps
1: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C
2: hydrogenchloride; water / tetrahydrofuran / 6 h / Reflux
With
hydrogenchloride; tetrabutyl ammonium fluoride; water;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C
2: hydrogenchloride / tetrahydrofuran / 6 h / Reflux
With
hydrogenchloride; tetrabutyl ammonium fluoride;
In
tetrahydrofuran;
|
-
![6-chloro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylene-cyclopentyl]-9H-purine-2-amine](/upload/2024/4/3a5b94e9-387f-4991-914a-7feadaff252c.png)
-
701278-58-2
6-chloro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylene-cyclopentyl]-9H-purine-2-amine

-

-
142217-69-4,76704-05-7
entecavir
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water;
at 70 ℃;
for 4h;
|
73% |
|
6-chloro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylene-cyclopentyl]-9H-purine-2-amine;
With
sodium hydroxide;
In
water;
at 72 ℃;
for 3.5h;
With
hydrogenchloride;
In
water;
at 0 ℃;
pH=6.3;
|
70% |
|
6-chloro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylene-cyclopentyl]-9H-purine-2-amine;
With
water; sodium hydroxide;
at 72 ℃;
for 3.5h;
With
hydrogenchloride;
In
water;
at 0 ℃;
pH=6.3;
|
70% |
|
With
hydrogenchloride; water;
In
tetrahydrofuran;
for 6h;
Reflux;
|
61% |
|
With
hydrogenchloride; water;
In
tetrahydrofuran;
for 6h;
Reflux;
|
61% |
|
With
hydrogenchloride;
In
tetrahydrofuran;
for 6h;
Reflux;
|
61% |
142217-69-4 Upstream products
-
142217-81-0
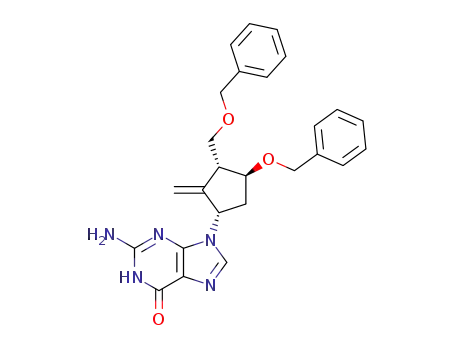
2-amino-1,9-dihydro-9-[(1S,3R,4S)-2-methylene-4-(phenylmethoxy)-3-[(phenylmethoxy)methyl]cyclopentyl]-6H-purin-6-one
-
39939-07-6
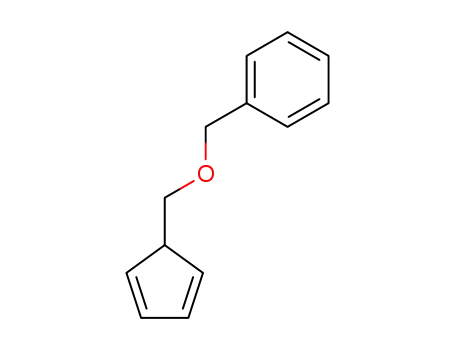
5-(benzyloxymethyl)cyclopentadiene
-
110567-22-1
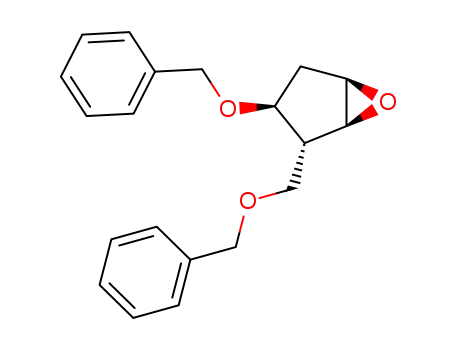
(2R,3S)-benzyloxy-2-benzyloxymethyl-6-oxabicyclo<3.1.0>hexane
-
117641-39-1
(2R,3S)-2-benzyloxymethyl-6-oxabicyclo<3.1.0>hexan-3-ol
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
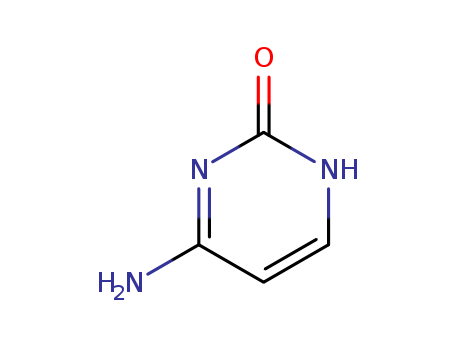
Cytosine
CAS:71-30-7
-
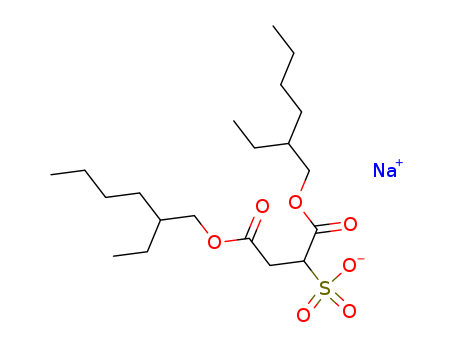
Dioctyl sulfosuccinate, sodium salt
CAS:577-11-7