55-22-1
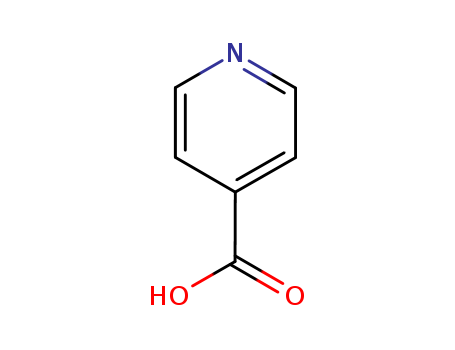
- Product Name:4-Picolinic acid
- Molecular Formula:C6H5NO2
- Purity:99%
- Molecular Weight:123.111
Product Details;
CasNo: 55-22-1
Molecular Formula: C6H5NO2
Appearance: white to light yellow crystal powder
Hot Sale Factory Supply High Purity 4-Picolinic acid 55-22-1 with Competitive Price
- Molecular Formula:C6H5NO2
- Molecular Weight:123.111
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:5.56E-07mmHg at 25°C
- Melting Point:≥300 °C(lit.)
- Refractive Index:1.5423 (estimate)
- Boiling Point:395.997 °C at 760 mmHg
- PKA:4.96(at 25℃)
- Flash Point:193.3 °C
- PSA:50.19000
- Density:1.293 g/cm3
- LogP:0.77980
Isonicotinic acid(Cas 55-22-1) Usage
|
Chemical Properties |
isonicotinic acid is also known as pyridine-4-carboxylic acid. white to light yellow crystal powder or white needle crystals. odorless, sublimable. molecular weight 123.11. melting point 319°C . slightly soluble in cold water, soluble in hot water, insoluble in alcohol, benzene and ether. isonicotinic acid is an amphoteric compound that is soluble in both acids and bases. soluble in hot water and ethanol, slightly soluble in cold water and ether. stable to heat and oxidation. |
|
Uses |
Isonicotinic acid is a metabolite of isoniazid. It is an isomer of nicotinic acid. The chronic toxicity of isonicotinic acid is slightly higher than that of isonicotinic acid hydrazide ( INH ) .Isonicotinic acid is a moderately basic compound (based on its pKa). Isonicotinic acid is an organic compound with a carboxyl group on a pyridine ring. The carboxyl group for isonicotinic acid is on the 4-position instead of the 3-position for nicotinic acid. It is an isomer of nicotinic acid. Isonicotinic acid is a metabolite of isoniazid.Isonicotinic acid considered to be inactive isomer of nicotinic acid. Isonicotinic acid is a metabolite of pyridine-4-carboxy hydrazide (isonicotinyl hydrazide; isoniazid) a front-line weapon in the battle against tuberculosis. Isonicotinic acid and its derivatives are used in manufacturing pharmaceuticals and agrochemicals.Used in the biological study of differentiation induced by nicotinic acid, nicotinamide and isonicotinic acid in human leukemia cell lines |
|
Definition |
ChEBI: Isonicotinic acid is a pyridinemonocarboxylic acid in which the carboxy group is at position 4 of the pyridine ring. It has a role as a human metabolite and an algal metabolite. It is a conjugate acid of an isonicotinate. |
|
Preparation |
Isonicotinic acid is synthesized by continuous oxidation with 4-methylpyridine as raw material and vanadium pentoxide as catalyst. The purity of the industrial product isonicotinic acid is more than 95%, and the yield of the above method is 70-75%. The consumption of 4-methylpyridine per ton of product is 1070kg. |
|
Application |
Isonicotinic acid (IN) can be used as:An organocatalyst in the one pot four component condensation reaction, to synthesize pyranopyrazoles based heterocyclic compounds.An organic ligand for the preparation of copper(I) halide coordination polymer [CuBr(IN)]n, by hydrothermal method.As a starting material for the synthesis of cation-dimers with potent antimalarial activity. |
|
Purification Methods |
Crystallise the acid repeatedly from water and dry it under vacuum at 110o or sublime it at 260o/15mm (m 319o). [Beilstein 22 III/IV 518, 22/2 V 188.] |
InChI:InChI=1/C6H5NO2/c8-6(9)5-1-3-7-4-2-5/h1-4H,(H,8,9)/p-1
55-22-1 Relevant articles
STABILITY OF WATER-SOLUBLE VITAMINS AND COENZYMES. VIII. KINETICS OF ACID HYDROLYSIS OF NICOTINOYL-γ-AMINOBUTYRIC ACID
Kozlov, E. I.,L'vova, M. Sh.,Garber, N. I.
, p. 328 - 333 (1988)
-
-
Lewis,Brown
, p. 890,892 (1944)
-
Continuous flow metal-free oxidation of picolines using air
Hamano, Masaya,Nagy, Kevin D.,Jensen, Klavs F.
, p. 2086 - 2088 (2012)
The metal free, direct oxidation of 2-, ...
Bench-scale biosynthesis of isonicotinic acid from 4-cyanopyridine by Pseudomonas putida
Zhu, Xiao-Yan,Gong, Jin-Song,Li, Heng,Lu, Zhen-Ming,Shi, Jin-Song,Xu, Zheng-Hong
, p. 739 - 744 (2014)
Pseudomonas putida CGMCC3830 harboring n...
-
Trubnikov et al.
, (1968)
-
Bimetallic cobalt-iron diselenide nanorod modified glassy carbon electrode: an electrochemical sensing platform for the selective detection of isoniazid
Sultan, Sundas,Zulqarnain, Muhammad,Shah, Afzal,Firdous, Naveeda,Nisar, Jan,Ashiq, Muhammad Naeem,Bakhsh, Esraa M.,Khan, Sher Bahadar
, p. 12649 - 12657 (2021)
The increasing demand of a sensitive and...
-
Bartok et al.
, p. 410 (1963)
-
Kinetics and mechanism of the reaction of iodine with isonicotinoylhydrazide
Funai,Blesa
, p. 2923 - 2928 (1984)
-
-
v.Euler,Hasselquist
, p. 439,445 (1958)
-
-
Afanas'eva et al.
, (1968)
-
-
Toma,Malin
, p. 288 (1975)
-
Assembly of three organic-inorganic hybrid supramolecular materials based on reduced molybdenum(V) phosphates
Zhang, He,Yu, Kai,Lv, Jing-Hua,Wang, Chun-Mei,Wang, Chun-Xiao,Zhou, Bai-Bin
, p. 22 - 30 (2014)
Three supramolecular materials based on ...
Decoration of copper foam with Ni nanorods and copper oxide nanosheets to produce a high-stability electrocatalyst for the reduction of CO2: Characterization of the electrosynthesis of isonicotinic acid
Mohammadzadeh, Safoora,Zare, Hamid R.,Khoshro, Hossein
, p. 678 - 685 (2019)
CuO–Cu2O (CuxO) nanosheets were coated o...
Communication-electrosynthesis of isonicotinic acid via indirect electrochemical reduction of pyridine in the presence of CO2
Ghobadi, Kobra,Zare, Hamid R.,Khoshro, Hossein,Gorji, Alireza
, p. H240 - H242 (2016)
The electrocatalytic reduction of CO2 by...
Kinetics of the highly selective liquid-phase oxidation of side chain alkyl groups in 2-methylpyrazine and picolines by selenium dioxide
Mukhopadhyay, Sudip,Chandalia, Sampatraj B.
, p. 455 - 459 (1999)
Kinetics of the liquid-phase oxidation o...
Aerobic oxidation of methylpyridines to pyridinecarboxylic acids catalyzed by N-hydroxyphthalimide
Shibamoto, Akihiro,Sakaguchi, Satoshi,Ishii, Yasutaka
, p. 505 - 508 (2000)
Selective aerobic oxidation of methylpyr...
Oxidation of Antitubercular Drug Isoniazid by a Lipopathic Oxidant, Cetyltrimethylammonium Dichromate: A Mechanistic Study
Garnayak, Sarita,Patel, Sabita
, p. 32 - 44 (2016)
The oxidation of an antitubercular drug ...
Thermostable amidase catalyzed production of isonicotinic acid from isonicotinamide
Mehta, Praveen Kumar,Bhatia, Shashi Kant,Bhatia, Ravi Kant,Bhalla, Tek Chand
, p. 1400 - 1404 (2015)
The biotransformation of isonicotinamide...
Oxidation of isoniazid by N-haloarenesulfonamidates in alkaline medium: A kinetic and mechanistic study
Puttaswamy,Anuradha,Ramachandrappa,Made Gowda
, p. 221 - 230 (2000)
The kinetics of oxidation of Isoniazid (...
Dual activity of electrocatalytic activated CO2 toward pyridine for synthesis of isonicotinic acid: An EC′C′C mechanism
Khoshro, Hossein,Zare, Hamid R.,Jafari, Abbas A.,Gorji, Alireza
, p. 69 - 71 (2015)
The present study demonstrates the indir...
An ESIPT-based colorimetric and fluorescent probe with large Stokes shift for the sensitive detection of hypochlorous acid and its bioimaging in cells
Ren, Haixian,Huo, Fangjun,Yin, Caixia
, p. 4724 - 4728 (2021)
Hypochlorous acid (HOCl), with a low phy...
Metabolism and pharmacokinetics of the cardiotonic agent piroximone and of its major metabolite in dog
Berg-Candolfi,Dulery,Jehl,Haegele
, p. 59 - 70 (1995)
1. Piroximone was administered orally (p...
SYNTHESIS OF PYRIDYL(TRICHLOROMETHYL)CARBINOLS UNDER INTERPHASE-CATALYSIS CONDITIONS
Iovel', I. G.,Gol'dberg, Yu. Sh.,Gaukhman, A. P.,Shimanskaya, M. V.
, p. 40 - 43 (1990)
The corresponding pyridyl(trichloromethy...
1,2-Dibutoxyethane-Promoted Oxidative Cleavage of Olefins into Carboxylic Acids Using O2 under Clean Conditions
Ou, Jinhua,Tan, Hong,He, Saiyu,Wang, Wei,Hu, Bonian,Yu, Gang,Liu, Kaijian
, p. 14974 - 14982 (2021/10/25)
Herein, we report the first example of a...
A decatungstate-based ionic liquid exhibiting a very low dielectric constant suitable for acting as a solvent and a catalyst for the oxidation of organic substrates
Martinetto, Yohan,Pégot, Bruce,Roch-Marchal, Catherine,Haouas, Mohamed,Cottyn-Boitte, Betty,Camerel, Franck,Jeftic, Jelena,Morineau, Denis,Magnier, Emmanuel,Floquet, Sébastien
, p. 9751 - 9755 (2021/06/15)
In this contribution, a new POM-based io...
Oxidation of Primary Alcohols and Aldehydes to Carboxylic Acids via Hydrogen Atom Transfer
Tan, Wen-Yun,Lu, Yi,Zhao, Jing-Feng,Chen, Wen,Zhang, Hongbin
supporting information, p. 6648 - 6653 (2021/09/08)
The oxidation of primary alcohols and al...
55-22-1 Process route
-
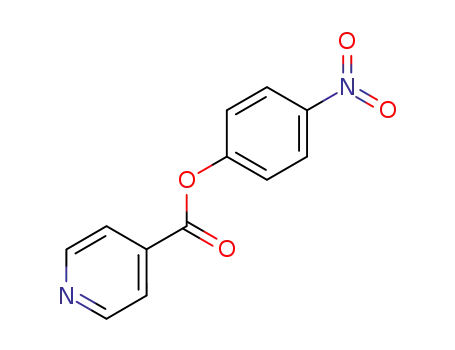
-
2882-35-1
isonicotinic acid 4-nitrophenyl ester

-
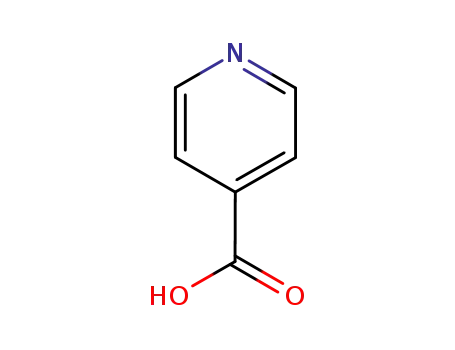
-
55-22-1
pyridine-4-carboxylic acid

-
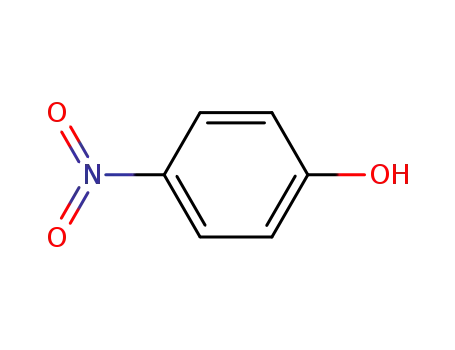
-
100-02-7,78813-13-5,89830-32-0
4-nitro-phenol
| Conditions | Yield |
|---|---|
|
With
N-(2-hydroxyethyl)piperazine-N'-(3-propanesulfonic acid) buffer;
<2-(3-hydroxybenzylamino)ethyl>3NH2Zn(II;
In
acetonitrile;
at 25 ℃;
Rate constant;
var. of catalyst, additives;
|
|
|
With
Cu complex of polymer from 2,6-bis-aminomethylpyridine and 4,4'-bis-aminomethyldiphenylmethane; water;
In
dimethyl sulfoxide;
at 25 ℃;
Rate constant;
var.reag.: Cu(2+) complex of 2,6-bis-benzylaminomethylpyridine; oligomers of 2,6-bis-aminomethylpyridine and 4,4'-bis-aminomethyldiphenylmethane;
|
-

-
2882-35-1
isonicotinic acid 4-nitrophenyl ester

-
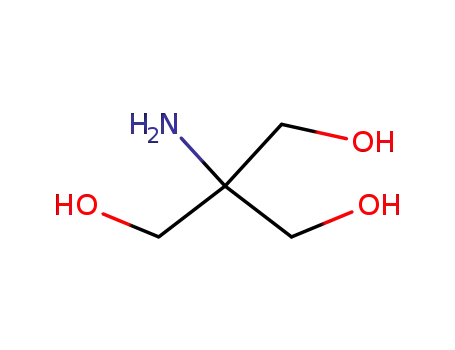
-
77-86-1
2-amino-2-hydroxymethyl-1,3-propanediol

-

-
55-22-1
pyridine-4-carboxylic acid

-

-
100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
-
90874-05-8
(HOCH2)3CNHCO(4-C5H4N)
| Conditions | Yield |
|---|---|
|
With
water;
at 25 ℃;
Rate constant;
|
55-22-1 Upstream products
-
100-43-6
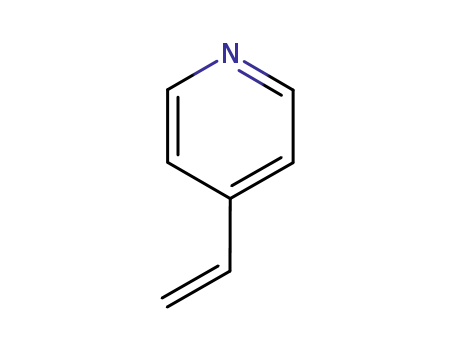
4-vinylpyridine
-
108-89-4
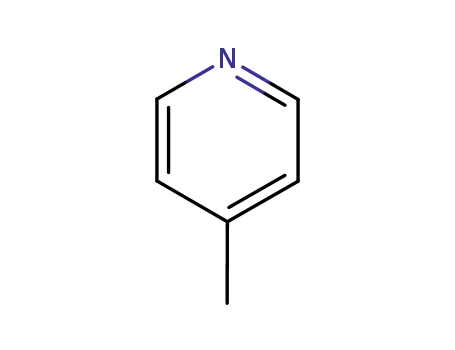
picoline
-
536-75-4
4-Ethylpyridine
-
108-75-8
2,4,6-trimethyl-pyridine
55-22-1 Downstream products
-
2459-09-8
4-pyridinecarboxylic acid, methyl ester
-
90610-01-8
n-propyl isonicotinate
-
1620-30-0
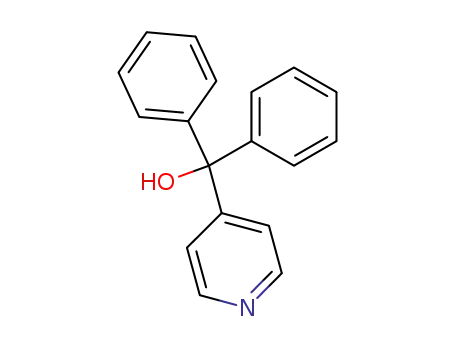
diphenyl(pyridin-4-yl)methanol
-
1570-45-2
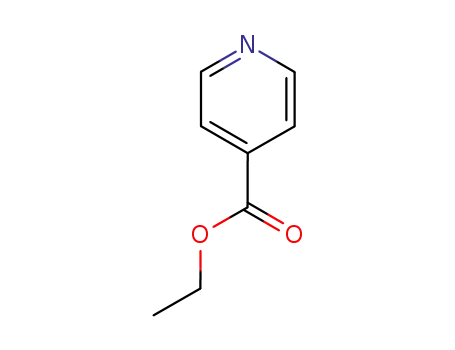
isonicotinic acid ethylester
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
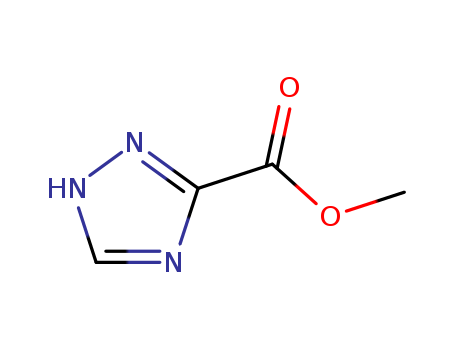
Methyl1,2,4-triazole-3-carboxylate
CAS:4928-88-5
-
Epinephrine bitartrate
CAS:51-42-3