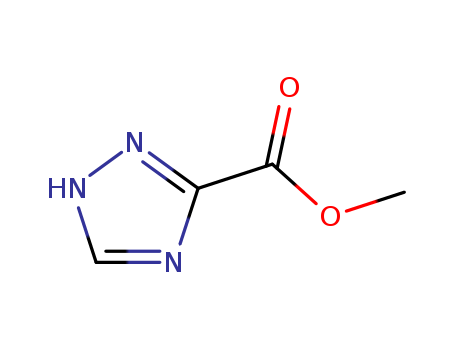
4928-88-5
- Product Name:Methyl1,2,4-triazole-3-carboxylate
- Molecular Formula:C4H5N3O2
- Purity:99%
- Molecular Weight:127.103
Product Details;
CasNo: 4928-88-5
Molecular Formula: C4H5N3O2
Appearance: white crystalline powder
Factory Sells 4928-88-5 In Stock, Factory Supply Methyl1,2,4-triazole-3-carboxylate
- Molecular Formula:C4H5N3O2
- Molecular Weight:127.103
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0.00307mmHg at 25°C
- Melting Point:196-199 °C(lit.)
- Refractive Index:1.534
- Boiling Point:283.9 °C at 760 mmHg
- PKA:7.96±0.20(Predicted)
- Flash Point:125.5 °C
- PSA:67.87000
- Density:1.38 g/cm3
- LogP:-0.40870
Methyl 1,2,4-triazole-3-carboxylate(Cas 4928-88-5) Usage
|
Uses |
Methyl 1,2,4-Triazole-3-carboxylate has been used as a reactant for the preparation of Ribavirin (1-β-D-ribofuranosyl)-1,2,4-triazole-3-carboxamide, an antiviral agent. |
|
Chemical Properties |
White solid |
|
General Description |
Methyl-1H-1,2,4-triazole-3-carboxylate can be synthesized from 5-amino-1,2,4-triazole-3-carboxylic acid via esterification with methanol. It is utilized as precursor for preparing the nucleoside analogue, Ribavirin. The crystal structure of methyl-1H-1,2,4-triazole-3-carboxylate has been analyzed. |
InChI:InChI=1/C4H5N3O2/c1-9-4(8)3-5-2-6-7-3/h2H,1H3,(H,5,6,7)
4928-88-5 Relevant articles
Synthesis process of ribavirin intermediate and the intermediate
-
Paragraph 0094-0096, (2020/08/12)
The invention discloses a synthesis proc...
Method for synthesizing 1, 2, 4-triazole-3-carboxylic acid methyl ester
-
Paragraph 0026-0027; 0030-0032; 0035-0037; 0040-0042; 0045, (2020/11/01)
The invention discloses a method for syn...
Structure Kinetics Relationships and Molecular Dynamics Show Crucial Role for Heterocycle Leaving Group in Irreversible Diacylglycerol Lipase Inhibitors
Janssen, Antonius P.A.,Van Hengst, Jacob M.A.,Béquignon, Olivier J.M.,Deng, Hui,Van Westen, Gerard J.P.,Van Der Stelt, Mario
, p. 7910 - 7922 (2019/10/11)
Drug discovery programs of covalent irre...
Preparation method for triazole derivative
-
Paragraph 0022-0026; 0027; 0028; 0029-0054; 0055-0072, (2018/11/27)
The invention discloses a preparation me...
4928-88-5 Process route
-
![5-amino-1H-[1,2,4]-triazole-3-carboxylic acid methyl ester](/upload/2024/4/66547e67-62c9-4703-9697-bfaae500960b.png)
- 3641-14-3
5-amino-1H-[1,2,4]-triazole-3-carboxylic acid methyl ester

-
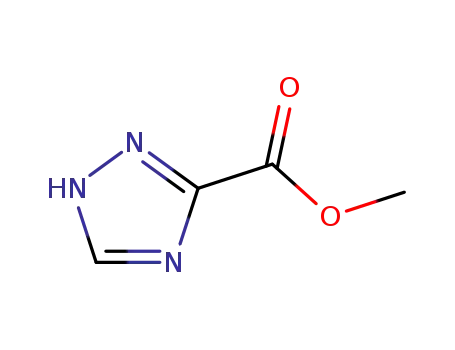
- 4928-88-5
methyl 1H-1,2,4-triazole-3-carboxylate
| Conditions | Yield |
|---|---|
|
With sodium hypophosphite; phosphoric acid; sodium nitrite; In water; at 0 - 20 ℃; for 1h; Reagent/catalyst; Temperature;
|
75% |
-
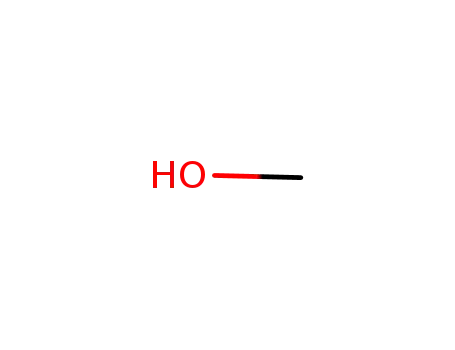
- 67-56-1
methanol

-
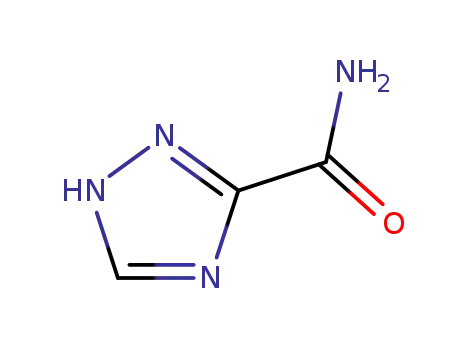
- 3641-08-5
1,2,4-triazole-3-carboxamide

-

- 4928-88-5
methyl 1H-1,2,4-triazole-3-carboxylate
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 80 ℃; for 4h;
|
4928-88-5 Upstream products
-
67-56-1

methanol
-
26663-10-5
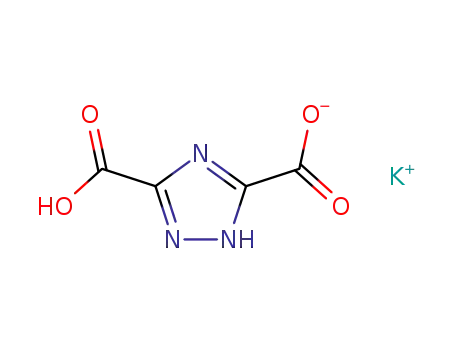
monopotassium salt of 1,2,4-triazole-3,5-dicarboxylic acid
-
4928-87-4
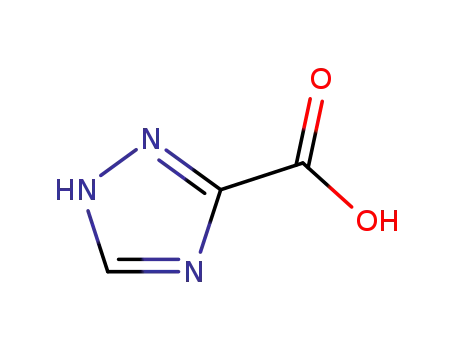
1,2,4-triazole-3-carboxylic acid
-
3641-14-3
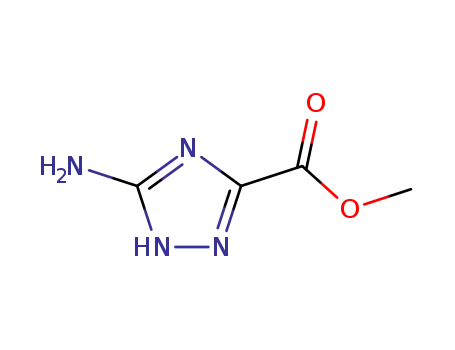
5-amino-1H-[1,2,4]-triazole-3-carboxylic acid methyl ester
4928-88-5 Downstream products
-
863609-11-4
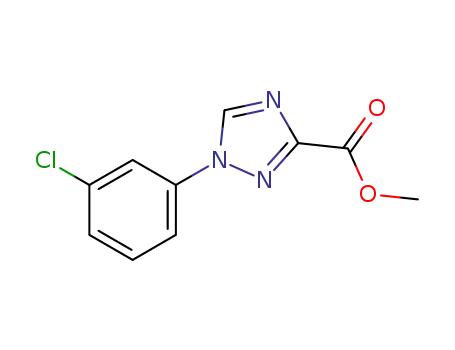
1-(3-chloro-phenyl)-1H-[1,2,4]-triazole-3-carboxylic acid methyl ester
-
143702-93-6
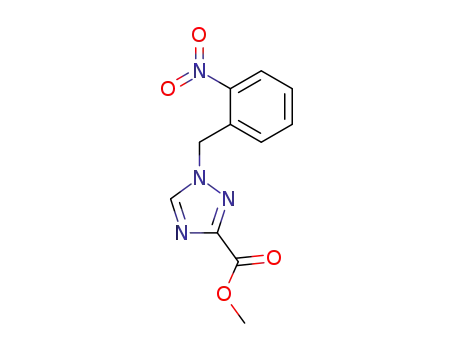
methyl 1-(2-nitrobenzyl)-1,2,4-triazole-3-carboxylate
-
143702-92-5
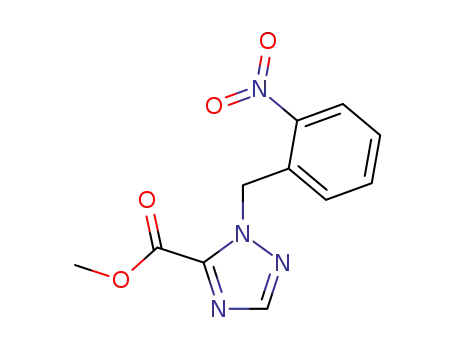
methyl 1-(2-nitrobenzyl)-1,2,4-triazole-5-carboxylate
-
123903-65-1
methyl 1-<<1,3(R)-bis(benzyloxy)-4-fluoro-2(R)-butoxy>methyl>-1,2,4-triazole-3-carboxylate
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
N-Chlorosuccinimide
CAS:128-09-6
-
4-Picolinic acid
CAS:55-22-1