65-45-2
- Product Name:Salicylamide
- Molecular Formula:C7H7NO2
- Purity:99%
- Molecular Weight:137.138
Product Details;
CasNo: 65-45-2
Molecular Formula: C7H7NO2
Appearance: white or light pink crystals or powder
Wholesale Chinese Manufacturer Supply Salicylamide 65-45-2 with Fast Shipping
- Molecular Formula:C7H7NO2
- Molecular Weight:137.138
- Appearance/Colour:white or light pink crystals or powder
- Vapor Pressure:0.000195mmHg at 25°C
- Melting Point:140-144 °C(lit.)
- Refractive Index:1.612
- Boiling Point:318.3 °C at 760 mmHg
- PKA:pKa 8.13(H2O t = 37) (Uncertain)
- Flash Point:146.3 °C
- PSA:63.32000
- Density:1.286 g/cm3
- LogP:1.19140
Salicylamide(Cas 65-45-2) Usage
|
Description |
Salicylamide is a white crystalline powder that is derived from salicylic acid and used as an analgesic and antipyretic. It is absorbed from the GI tract on oral administration and is rapidly metabolized to inactive metabolites by intestinal mucosa, but not by hydrolysis. Activity appears to reside in the intact molecule. Salicylamide is approximately 40 to 55% plasma protein bound, and it competes with other salicylates and acetaminophen for glucuronide conjugation, decreasing the extent of conjugation of these other drugs. It is also excreted much more rapidly than other salicylates,which accounts for its lower toxicity. It is available inseveral nonprescription products, in combination with acetaminophenand phenyltoloxamine (e.g., Rid-A Pain compound,Cetazone T, Dolorex, Ed-Flex, Lobac) or with aspirin,acetaminophen, and caffeine (e.g., Saleto, BC Powder). |
|
Uses |
Medicine (analgesic). Whereas salicylamide is reported to be as effective as aspirin as an analgetic/antipyretic and is effective in relieving pain associated with arthritic conditions, it does not appear to possess useful anti-inflammatory activity. Thus, indications for the treatment of arthritic disease states are unwarranted, and its use is restricted to the relief of minor aches and pain at a dosage of 325 to 650 mg three or four times per day. Its effects in humans are not reliable, however, and its use is not widely recommended. |
|
Definition |
ChEBI: The simplest member of the class of salicylamides derived from salicylic acid. |
|
Air & Water Reactions |
Salicylamide darkens on exposure to air. . Insoluble in water. |
|
Fire Hazard |
Flash point data for Salicylamide are not available; however, Salicylamide is probably combustible. |
|
Purification Methods |
Crystallise the amide from water or repeatedly from CHCl3 [Nishiya et al. J Am Chem Soc 108 3880 1986]. [Beilstein 10 IV 169.] The anilide [87-17-2] M 213.2, m 135o crystallises from H2O. [Beilstein 12 H 500, 12 I 268, 12 II 256, 12 944.] |
InChI:InChI=1/C7H7NO2/c8-7(10)5-3-1-2-4-6(5)9/h1-4,9H,(H2,8,10)
65-45-2 Relevant articles
Copper-mediated α-hydroxylation of N-salicyloyl-glycine. A model for peptidyl-glycine α-amidating monooxygenase (PAM)
Capdevielle, Patrice,Maumy, Michel
, p. 3831 - 3834 (1991)
Title compound 1 is selectively hydroxyt...
Mg/Al mixed oxides: Heterogeneous basic catalysts for the synthesis of salicylamide from urea and phenol
Wang, Dengfeng,Zhang, Xuelan,Wei, Wei,Sun, Yuhan
, p. 159 - 162 (2012)
Several Mg/Al mixed oxides were prepared...
Green and efficient Beckmann rearrangement by Cu(II) contained nano-silica triazine based dendrimer in water
Bahreininejad, Mohammad Hasan,Moeinpour, Farid
, p. 893 - 901 (2021/01/12)
In this research, a Cu(II) contained nan...
Pharmacological and toxicological studies on salicylamide
C. T. Ichniowski, W. C. Hueper
Journal of the American Pharmaceutical Association, Volume35, Issue8 August 1946 Pages 225-230
The acute and chronic toxicities, pharmacologic and hematologic effects of salicylamide have been studied. Aspirin served as a comparative control. Salicylamide exerts a moderately quicker and deeper depressing effect than aspirin. Oral daily feeding of one-fourth the single lethal dose for a period of thirteen weeks did not induce any untoward symptomatic and anatomic reactions. Data presented suggest that salicylamide appears to differ in its metabolization from other salicylic compounds. The oral lethal dose of salicylamide in rats equals that of aspirin.
Asymmetric Hydrogenation of Cationic Intermediates for the Synthesis of Chiral N,O-Acetals
Sun, Yongjie,Zhao, Qingyang,Wang, Heng,Yang, Tilong,Wen, Jialin,Zhang, Xumu
supporting information, p. 11470 - 11477 (2020/08/10)
For over half a century, transition-meta...
65-45-2 Upstream products
-
2037-95-8
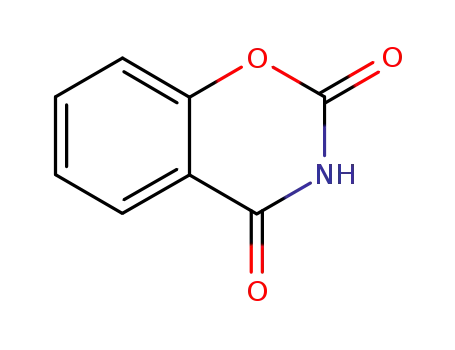
1,3-benzoxazine-2,4-dione
-
62-53-3
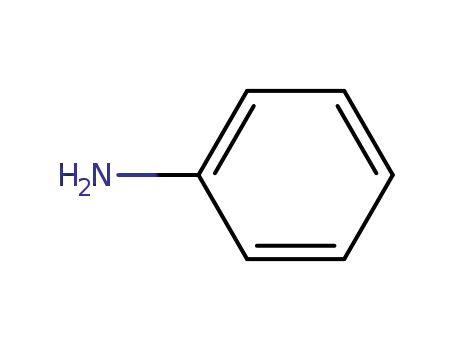
aniline
-
20602-57-7
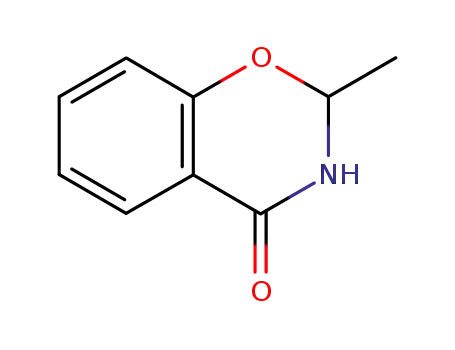
2-methyl-2,3-dihydro-4H-1,3-benzoxazin-4-one
-
91132-80-8
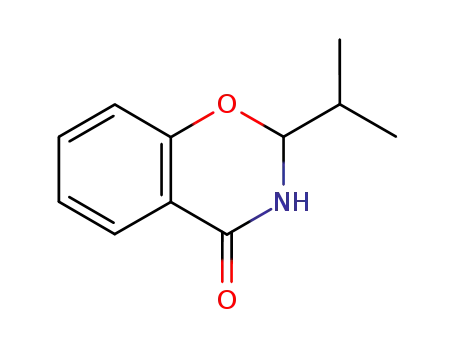
2-isopropyl-2,3-dihydro-4H-benzo[e][1,3]oxazin-4-one
65-45-2 Downstream products
-
1144-30-5
2-hydroxy-N-piperidin-1-ylmethyl-benzamide
-
17357-04-9
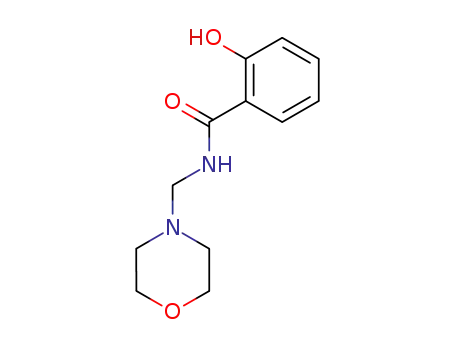
2-hydroxy-N-morpholin-4-ylmethyl-benzamide
-
13316-79-5
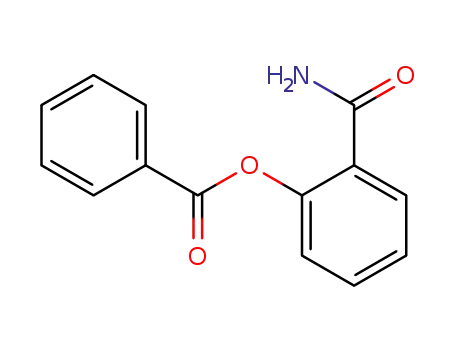
O-Benzoylsalicylamide
-
132305-19-2
benzoyl-(2-benzoyloxy-benzoyl)-amine
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
Allopurinol
CAS:315-30-0
-
3,5,6-Trichlorosalicylic acid
CAS:40932-60-3