105816-04-4
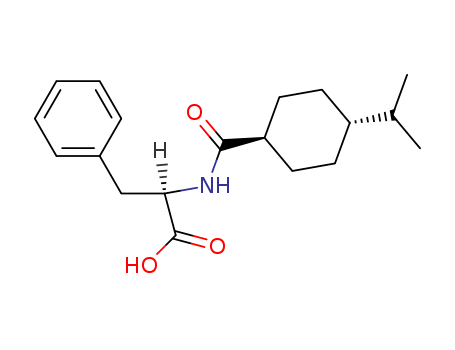
- Product Name:Nateglinide
- Molecular Formula:C19H27NO3
- Purity:99%
- Molecular Weight:317.428
Product Details;
CasNo: 105816-04-4
Molecular Formula: C19H27NO3
Appearance: white crystalline powder
Hot Sale Trustworthy Manufacturer Supply Nateglinide 105816-04-4 with Reasonable Price
- Molecular Formula:C19H27NO3
- Molecular Weight:317.428
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:137-141 °C
- Refractive Index:1.536
- Boiling Point:527.6 °C at 760 mmHg
- PKA:3.61±0.10(Predicted)
- Flash Point:272.9 °C
- PSA:66.40000
- Density:1.104 g/cm3
- LogP:3.65180
Nateglinide(Cas 105816-04-4) Usage
|
Physical and Chemical Properties |
White or almost white crystalline powder, odorless, bitter taste. It is soluble in methanol, ethanol, chloroform, dissolved in acetone, ethyl ether, almost insoluble in water. Valid form used in clinic is H-type, mp 137~141 ℃. [Α] D-37.5 °, the maximum UV absorption wavelength in methanol is 252,257,263nm. The above information is edited by the lookchem of Tian Ye. |
|
Hypoglycemic agents |
Nateglinide and mitiglinide, repaglinide are three commonly used non-sulfonylurea oral hypoglycemic agents for insulin secretion,it is successfully developed for the first time by the Japanese company Ajomoto , its chemical structure belongs to carbamoylmethyl-benzoic acid (CMBA), it belongs to D-phenylalanine derivatives, it is a new generation of antidiabetic drugs having amino acid structure ,it is an amino acid derivative prompting insulin secretion, it is also currently the only non-sulfonylureas insulinotropic agent having amino acid structure. The mechanism is mainly through binding the pancreatic β cell sulfonylurea receptor, blocking islet cell ATP-sensitive potassium channels, leading to membrane depolarization, causing the calcium channe lopen to promote insulin secretion. This product is a new type of meal blood glucose regulator, which can effectively control the postprandial blood glucose levels, with rapid onset, short duration of action, low incidence of cardiovascular side effects and hypoglycemia and other characteristics. Oral bioavailability is 72%, after 15min it can produce insulin secretion effect, Tmax is 0.5~0.9 h, 0.2 h insulin levels achieve peak ,after 1.5 h it is similar to placebo. The plasma protein binding rate is 99%. After reaching plasmapeak , plasma concentrations decline rapidly.T1/2 of oral administration of 120 mg and intravenous injection 60 mg of is 1.5 to 1.7 hours, and the plasma clearance is 7.4 hours. It is metabolized in the liver by isoenzyme CYP2C9 and CYP3A4 way. The main metabolites are products after isomeric oxidation,it can be hydroxyl, diastereomers, isopropyl isomer or unsaturated aliphatic isomers.The main metabolites in plasma and urine are metabolites after the hydroxylation of isopropyl methine family (me-thine carbon) . About 2/3 nateglinide is excreted from the fecal , and the rest is excreted in the urine. Such excretion way is beneficial to elderly Ⅱ diabetes mellitus with renal dysfunction. nateglinide eliminate T1/2 is 1.4 h, because nateglinide T1/2 is short, there is no report yet about the drug accumulation in the body . Long-term use of nateglinide does not produce drug-induced hypoglycemia caused by drug accumulation .It is used for the treatment of diet therapy, exercise therapy and mild to moderate non-insulin dependent (Ⅱ type) diabetes which taking α-glucosidase inhibitor can not control. The results show that: nateglinide can be used more physiologically for meal glucose control, there is less opportunity for contact with insulin and hypoglycemia which allows patients to flexibly plan scheduling mealtimes, which means there is medication while eating, not eating no medication. |
|
Uses |
It is used for the treatment of diabetes |
|
Description |
Nateglinide is a N-acylated D-phenylalanine marketed in Japan as novel orally active insulinotropic agent for the treatment of type-2 diabetes mellitus. It belongs to the class of nonsulfonylureas and shows some structural similarity to repaglinide, the only other representative in this family. In single pancreatic beta-cells isolated from rats, Nateglinide was found to specifically block the ATP-sensitive K+ channel resulting in an increase in intracellular calcium concentration. This primary action would underlie the mechanism by which Nateglinide markedly stimulates or potentiates, depending on glucose concentrations, insulin secretion from pancreatic beta-cells. Clinical studies demonstrated a good safety profile with a low potential for hypoglycemia. The pharmacokinetic profile was consistent with the changes of the blood glucose and plasma insulin level. Interestingly, Nateglinide exerts a rapid onset and short duration of action due to a rapid absorption and clearance. Unlike other similar agents, Nateglinide suppresses postprandial glucose elevations. |
|
Chemical Properties |
Cyrstalline Solid |
|
Originator |
Ajinomoto (Japan) |
|
Definition |
ChEBI: An N-acyl-D-phenylalanine resulting from the formal condensation of the amino group of D-phenylalanine with the carboxy group of trans-4-isopropylcyclohexanecarboxylic acid. An orally-ad inistered, rapidly-absorbed, short-acting insulinotropic agent, it is used for the treatment of type 2 diabetes mellitus. |
|
Brand name |
Starlix (Novartis);Fastic;Starsis. |
|
General Description |
Although nateglinide, N-(4-isopropylcyclohexanecarbonyl)-D-phenylalanine (Starlix), belongs tothe metaglinides, it is a phenylalanine derivative and representsa novel drug in the management of type 2 diabetes. |
|
Biochem/physiol Actions |
Nateglinide is a Kir6.2/SUR1 channel inhibitor and antidiabetic. It is selective for the SUR1 subtype, which is found on pancreatic islet cells. Nateglinide evokes KATP channel-dependent insulin secretion (50-200 μM) in the absence and presence of insulin. |
|
Mechanism of action |
Approved in the United States in late 2000, nateglinide is a rapidly absorbed insulin secretagogue that has a mechanism of action similar to that of repaglinide, with effects appearing within 20 minutes following oral dosing. Bioavailability is 73%, and it is 98% protein bound, primarily to albumin. Nateglinide is tissue selective, with low affinity for cardiac and skeletal muscle. |
|
Clinical Use |
Treatment of type 2 diabetes in combination with metformin |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: concentration reduced by rifampicin. Antifungals: hypoglycaemic effect possibly enhanced by fluconazole. Lipid-lowering agents: hypoglycaemic effect possibly enhanced by gemfibrozil. |
|
Metabolism |
It is metabolized in the liver, with 16% excreted in the urine unchanged. The major metabolites are hydroxyl derivatives (CYP2C9, 70%; CYP3A4, 30%) that are further conjugated to the glucuronide derivatives. |
InChI:InChI=1/C19H27NO3/c1-13(2)15-8-10-16(11-9-15)18(21)20-17(19(22)23)12-14-6-4-3-5-7-14/h3-7,13,15-17H,8-12H2,1-2H3,(H,20,21)(H,22,23)/t15-,16-,17-/m1/s1
105816-04-4 Relevant articles
METHODS FOR TREATING CHRONIC FATIGUE SYNDROME AND MYALGIC ENCEPHALOMYELITIS
-
, (2021/03/13)
In one aspect the invention relates to a...
A high-purity process for the preparation of nateglinide
-
Paragraph 0028; 0030-0032, (2017/05/04)
A preparation method of high-purity nate...
A PROCESS FOR THE PREPARATION OF NATEGLINIDE
-
Page/Page column 22-23, (2012/01/06)
The present invention relates to a proce...
A PROCESS FOR THE PREPARATION OF N-[[TRANS-4-(1-METHYLETHYL)CYCLOHEXYL]CARBONYL]-D-PHENYLALANINE
-
Page/Page column 17-18, (2010/10/03)
The present invention is directed to an ...
105816-04-4 Process route
-
-
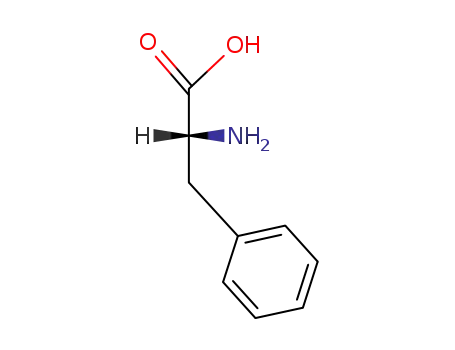
673-06-3,25191-15-5,658-69-5,67675-33-6
D-(R)-phenylalanine

-
-
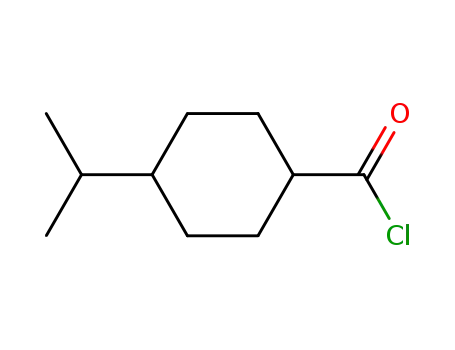
100597-38-4
4-iso-propylcyclohexanecarbonyl chloride

-
-
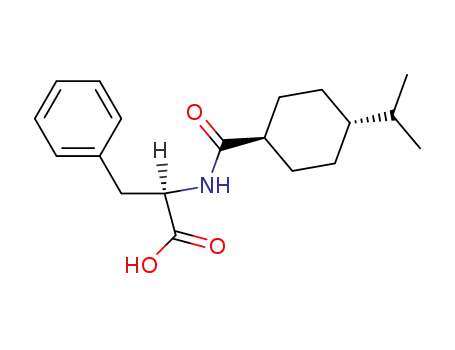
105816-04-4,105816-05-5
nateglinide
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide; sulfuric acid;
|
65% |
|
With
sodium hydroxide; sulfuric acid;
In
n-heptane; ethyl acetate;
|
65% |
|
With
potassium hydroxide;
In
water; acetone;
|
-
-
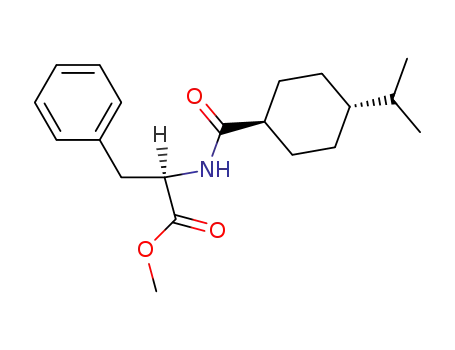
105746-47-2
N-<(trans-4-Isopropylcyclohexyl)carbonyl>-D-phenylalanine methyl ester

-

-
105816-04-4,105816-05-5
nateglinide
| Conditions | Yield |
|---|---|
|
N-<(trans-4-Isopropylcyclohexyl)carbonyl>-D-phenylalanine methyl ester;
With
water; sodium hydroxide;
In
methanol;
at 25 - 30 ℃;
for 5h;
With
hydrogenchloride;
In
water;
pH=2.0 - 2.5;
|
80.8% |
|
With
sodium hydroxide;
In
methanol;
Yield given;
|
|
|
N-<(trans-4-Isopropylcyclohexyl)carbonyl>-D-phenylalanine methyl ester;
With
sodium hydroxide;
In
acetone;
at 20 - 25 ℃;
for 2h;
With
hydrogenchloride;
In
water;
at 10 ℃;
for 1h;
pH=2 - 3;
Product distribution / selectivity;
|
105816-04-4 Upstream products
-
105746-47-2

N-<(trans-4-Isopropylcyclohexyl)carbonyl>-D-phenylalanine methyl ester
-
7077-05-6
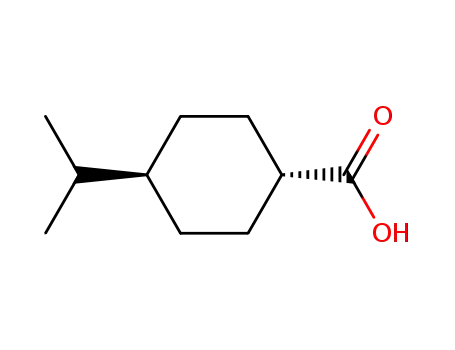
trans-4-isopropylcyclohexane-1-carboxylic acid
-
673-06-3

D-(R)-phenylalanine
-
541-41-3
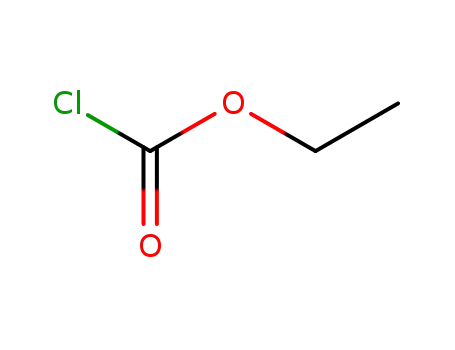
chloroformic acid ethyl ester
105816-04-4 Downstream products
-
187728-85-4
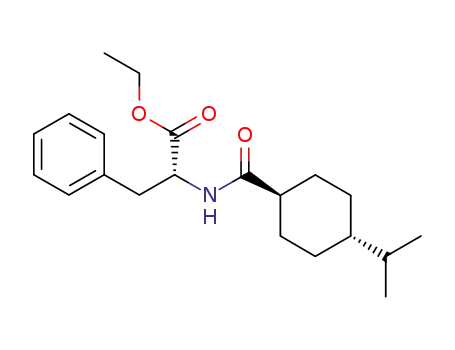
ethyl ((1r,4R)-4-isopropylcyclohexane-1-carbonyl)-D-phenylalaninate
-
105746-47-2

N-<(trans-4-Isopropylcyclohexyl)carbonyl>-D-phenylalanine methyl ester
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
2'-Deoxyuridine
CAS:951-78-0
-
Benzathine Cloxacillin
CAS:23736-58-5