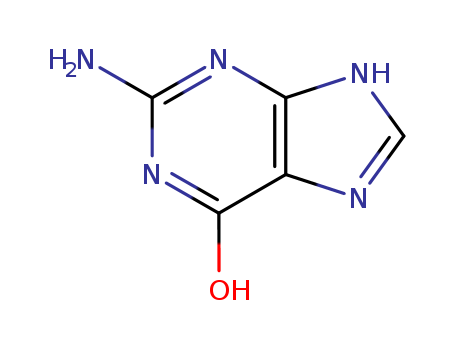
Product Details;
CasNo: 73-40-5
Molecular Formula: C5H5N5O
Appearance: white to light yellow crystal powder
Trustworthy Factory Supply High Purity Guanine 73-40-5 with Safe Transportation
- Molecular Formula:C5H5N5O
- Molecular Weight:151.128
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:5.86E-14mmHg at 25°C
- Melting Point:>300 °C(lit.)
- Refractive Index:2.047
- Boiling Point:591.4oC at 760 mmHg
- PKA:9.92(at 40℃)
- Flash Point:311.4oC
- PSA:100.45000
- Density:2.196 g/cm3
- LogP:-0.19040
Guanine(Cas 73-40-5) Usage
|
Organic bases |
Guanine is an organic base that is widespread in the animal and the plant kingdom. The chemical name is 2-amino-6-oxo-purin. It is colorless square crystals or crystalline powder. In the aqueous solution containing a large excess of ammonia, it will become small diamond crystal after slowly evaporating the water. It has a melting point of 360 ℃ (partially decomposed and sublimated). It can be dissolved in ammonia water, caustic soda and dilute mineral acid, slightly soluble in ethanol, ethyl ether, and insoluble in water. It has a strong UV absorption. It is the major composition of guanosine and guanylate. Its hydrochloride monohydrate is powdery crystals with water being loss at 100 ℃ and hydrogen chloride being loss at 200 ℃. It is colorless needle crystal or amorphous powder. The melting point is 360 ℃ (decomposition). It is easily soluble in acid and alkali, slightly soluble in alcohol, ether, but insoluble in water. The above information is edited by the lookchem of Dai Xiongfeng. |
|
6-Thioguanine |
6-Thioguanine belongs to another kind of common purine metabolism antagonist in the inhibition of the purine synthesis pathway and is a cell cycle specific drugs to which those cells locating in the cycle S period are most sensitive. In addition to inhibit the biosynthesis of cellular DNA, it also has mild inhibitory effect on the biosynthesis of RNA. This product is a kind of guanosine analogs. It becomes active only after being converted to 6-TG ribonucleotide via the phosphor-ribosyltransferase inside human body. The action process of this product is similar to that of mercapto-purine. In addition, 6-TG ribonucleotide, through its inhibitory effect on guanylate kinase, can prevent the phosphorylation of guanosine monophosphate (GMP) into guanosine diphosphate (GDP). This product, after being metabolized into deoxyribonucleoside triphosphate, can be embedded in DNA, thus further inhibiting the biosynthesis of nucleic acids while mercaptopurine having no effect. The product has cross-resistance with mercaptopurine while it can have its efficacy improved upon combination with other drugs such as cytarabine. Its oral absorption after oral administration is incomplete, at about 30%. Only a relatively small amount of the drug can shift from the blood to penetrate through the blood-brain barrier, therefore at generally oral dose, it is insufficient to prevent and treat meningeal leukemia. The activation and decomposition process of the product both proceeds in the liver with being de-activated through either shifting the amino-methyl mercaptopurine via methylation or shifting to mercaptopurine via deamination. However, the metabolic process of inactivation is not related to xanthine oxidase, therefore taking allopurinol has no significant inhibitory effect on the metabolism of this product. The half-life of intravenous injection is 25~240 min with the average period being 80 min. Through being excreted through the kidneys, for one time of oral administration, about 40% of the drug is excreted in urine in the form of metabolites within 24h with only trace amount of 6-Thioguanine being detected in the urine. |
|
Uses |
Guanine is one of the two purines comprising the five nucleic acid bases. Much of the information regarding the general role of nucleic acid bases is covered in Adenine and Cytosine. Guanine gets its name from guano, from which it was first isolated in the 1840s. Albrecht Kossel (1853 1927) determined that guanine (as well as adenine, cytosine, thymine and uracil) was a component of nucleic acid in the last two decades of the 19th century. Similar to adenine, guanine combines with ribose to form a nucleoside. The nucleoside produced is guanosine, which in turn combines with one to three phosphoryls to yield the nucleotides guanosine monophosphate (GMP), guanosine diphosphate (GDP), and guanosine triphosphate (GTP), respectively. Guanine nucleotides play an important role in metabolism including the conversion of adenosine diphosphate (ADP) to adenosine triphosphate (ATP) and carbohydrate metabolism. It can be used for biochemical studies. It can be used as the intermediates of the antiviral drugs acyclovir. It can be used as the intermediate of thioguanine and open-ringed guanine. |
|
Production method |
5-amino-4-imidazolyl amide can have esterification reaction with isothiocyanate methylbenzene to generate ester, and then successfully reacted with methyl iodide, ammonia to synthesize it. |
|
Definition |
A nitrogenous base found in DNA and RNA. Guanine has a purine ring structure. |
|
Biological Activity |
guanine is one of the four main nucleobases found in the nucleic acids dna and rna.guanine is a purine derivative, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds. |
InChI:InChI=1/C5H5N5O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H4,6,7,8,9,10,11)
73-40-5 Relevant articles
Origin of difference between one-electron redox potentials of guanosine and guanine: Electrochemical and quantum chemical study
Langmaler, Jan,Samec, Zdeneì?k,Samcovaì?, Eva,Hobza, Pavel,Rì?eha, David
, p. 15896 - 15899 (2004)
Cyclic voltammetry was used to measure t...
Kinetics of hydrolysis of 8-(arylamino)-2′-deoxyguanosines
Novak, Michael,Ruenz, Megan,Kazerani, Shahrokh,Toth, Krisztina,Nguyen, Thach-Mien,Heinrich, Brian
, p. 2303 - 2308 (2002)
The 8-(arylamino)-2′-deoxyguanosines, or...
π-Interactions of modified nucleobases. On mesomeric purine betaines with inversed charge properties
Schmidt, Andreas,Karl Kindermann, Markus
, p. 2379 - 2384 (2001)
Intermolecular interactions of modified ...
REACTION OF GUANOSINE DERIVATIVES WITH PHOSPHORUS TRICHLORIDE IN ACETONE
Honjo, Mikio,Maruyama, Tokumi,Sato, Sumiko,Marumoto, Ryuji
, p. 2663 - 2666 (1981)
2',3'-Di-O-protected guanosine derivativ...
Novel use of a guanosine prodrug approach to convert 2′,3′-didehydro-2′,3′-dideoxyguanosine into a viable antiviral agent
Ray, Adrian S.,Yang, Zhenjun,Chu, Chung K.,Anderson, Karen S.
, p. 887 - 891 (2002)
Transient kinetic studies with human imm...
Physicochemical properties of carbovir, a potential anti-HIV agent
Anderson,Chiang
, p. 787 - 790 (1990)
(±)-Carbovir [(±)-9-[4α-(hydroxymethyl)-...
THE EFFECT OF METAL ION COMPLEX FORMATION ON ACIDIC DEPURINATION OF 2'-DEOXYADENOSINE AND 2'-DEOXYGUANOSINE
Arpalahti, Jorma,Kaeppi, Rainer,Hovinen, Jari,Loennberg, Harri,Chattopadhyaya, Jyoti
, p. 3945 - 3954 (1989)
The substitution inert N7-(dien)Pt(II) c...
Nitrogen-Doped Carbon Supported Co/Ni Bimetallic Catalyst for Selectively Reductive N-Formylation of Nitroso in Guanine Synthesis
Liu, Peng,Shi, Dongxu,Wang, Ke,Zhang, Hong-yu,Zhang, Yuecheng,Zhao, Jiquan
, (2021/11/22)
A nitrogen-doped carbon supported Co/Ni ...
Thermodynamic Reaction Control of Nucleoside Phosphorolysis
Kaspar, Felix,Giessmann, Robert T.,Neubauer, Peter,Wagner, Anke,Gimpel, Matthias
supporting information, p. 867 - 876 (2020/01/24)
Nucleoside analogs represent a class of ...
Sources of 2,5-diaminoimidazolone lesions in DNA damage initiated by hydroxyl radical attack
Thomas, Caroline Suzanne,Pollard, Hannah Catherine,Razskazovskiy, Yuriy,Roginskaya, Marina
, p. 517 - 524 (2020/09/07)
The present study reports radiation-chem...
Method for synthesizing guanine by guanosine hydrolysis method (by machine translation)
-
Paragraph 0031-0070, (2020/08/02)
The invention belongs to the field of ch...
73-40-5 Upstream products
-
64-18-6
formic acid
-
1004-75-7
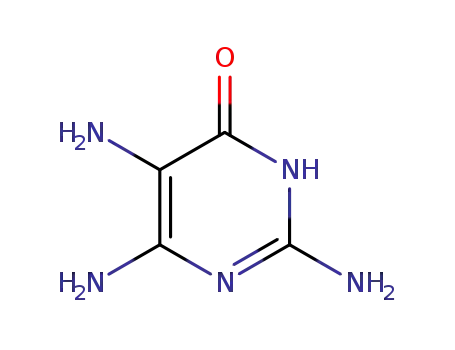
2,5,6-triamino-3,4-dihydro-4-pyrimidinone
-
141-53-7
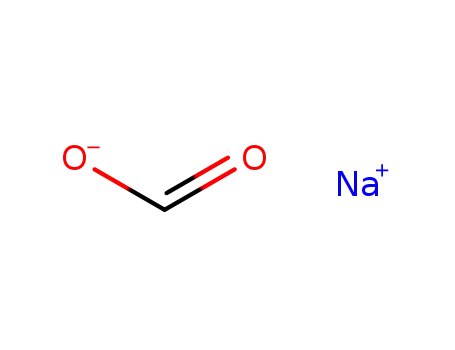
sodium formate
-
122-51-0
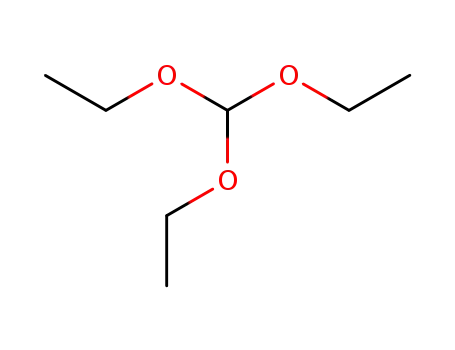
orthoformic acid triethyl ester
73-40-5 Downstream products
-
961-07-9
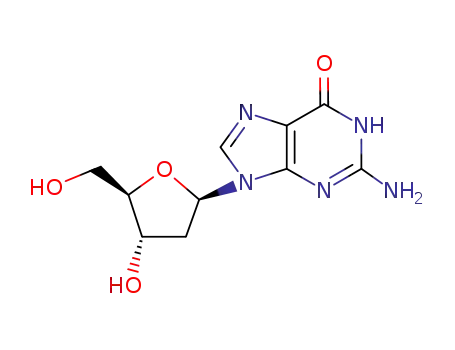
2'-Deoxyguanosine
-
26758-00-9
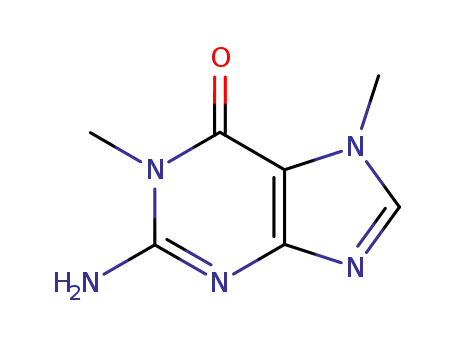
1,7-dimethylguanine
-
28128-41-8
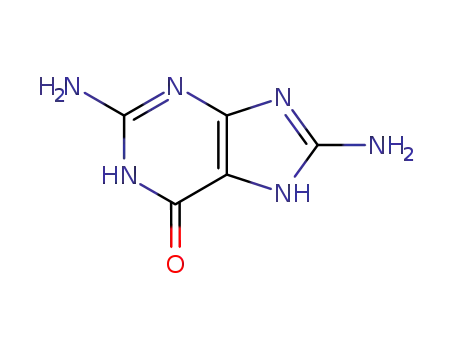
8-aminoguanine
-
75056-37-0
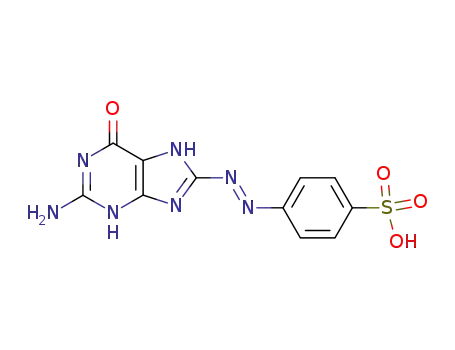
4-(2-amino-6-oxo-6,7-dihydro-3H-purin-8-ylazo)-benzenesulfonic acid
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
BENZYL 4-BENZYLOXYBENZOATE
CAS:56442-22-9
-
5,5-Dimethylhydantoin
CAS:77-71-4