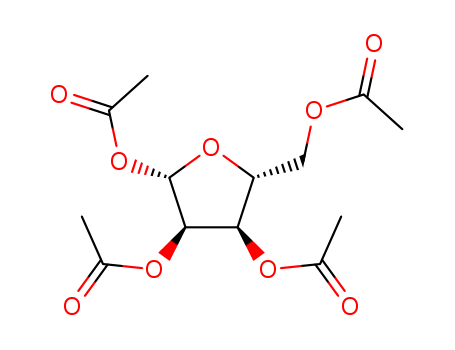
13035-61-5
- Product Name:1,2,3,5-Tetra-O-Acetyl-D-Ribose
- Molecular Formula:C13H18O9
- Purity:99%
- Molecular Weight:318.281
Product Details;
CasNo: 13035-61-5
Molecular Formula: C13H18O9
Appearance: white to almost white crystalline powder
Quality Manufacturer Supply 99% Pure 1,2,3,5-Tetra-O-Acetyl-D-Ribose 13035-61-5 with Safe Delivery
- Molecular Formula:C13H18O9
- Molecular Weight:318.281
- Appearance/Colour:white to almost white crystalline powder
- Vapor Pressure:3.76E-06mmHg at 25°C
- Melting Point:81-83 °C(lit.)
- Refractive Index:-14.5 ° (C=5, MeOH)
- Boiling Point:385.6 °C at 760 mmHg
- Flash Point:168.5 °C
- PSA:114.43000
- Density:1.29 g/cm3
- LogP:-0.29910
beta-D-Ribofuranose 1,2,3,5-tetraacetate(Cas 13035-61-5) Usage
|
Chemical Properties |
beta-D-Ribofuranose 1,2,3,5-tetraacetate is white to almost white crystalline powder |
|
Uses |
beta-D-Ribofuranose 1,2,3,5-tetraacetate is used in the synthesis of 3-(β-D-ribofuranosyl)-2,3-dihydro-6H-1,3-oxazine-2,6-dione, a new pyrimidine nucleoside analog related to uridine. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C13H18O9/c1-6(14)18-5-10-11(19-7(2)15)12(20-8(3)16)13(22-10)21-9(4)17/h10-13H,5H2,1-4H3/t10-,11?,12?,13-/m1/s1
13035-61-5 Relevant articles
A facile synthesis of 9-(1,3-dihydroxy-2-propoxymethyl)guanine (ganciclovir) from guanosine
Boryski, Jerzy,Golankiewicz, Bozenna
, p. 625 - 628 (1999)
The potent and selective antiviral drug ...
-
Viscontini et al.
, p. 1373,1375 (1954)
-
An unusual transformation of isometric forms of tetra acetyl D-ribofuranose.
DAVOLL,BROWN,VISSER
, p. 64 - 65 (1952)
-
Syntheses of 1-thio-D-xylose and D-ribose esters of diorganoarsinous acids and their anticancer activity
Gao, Mingzhang,Chen, Yiwen,Tan, Songde,Reibenspies, Joseph H.,Zingaro, Ralph A.
, p. 199 - 206 (2008)
Several thio-D-xylose and D-ribose ester...
A Practical and Convenient Method for Utilization of the Mother Liquors Containing 1,2,3,5-Tetra-O-Acetyl-α-D-Ribofuranose
Mei,Guan,Li
, p. 592 - 596 (2018)
-
-
Chittenden
, p. 491 (1972)
-
Synthesis of 1,2,3-triazolyl nucleoside analogues and their antiviral activity
Andreeva, Olga V.,Garifullin, Bulat F.,Zarubaev, Vladimir V.,Slita, Alexander V.,Yesaulkova, Iana L.,Saifina, Liliya F.,Shulaeva, Marina M.,Belenok, Maya G.,Semenov, Vyacheslav E.,Kataev, Vladimir E.
, p. 473 - 490 (2020/09/22)
Abstract: Based on the fact that a searc...
Synthesis and antiviral evaluation of nucleoside analogues bearing one pyrimidine moiety and two d-ribofuranosyl residues
Andreeva, Olga V.,Belenok, Mayya G.,Garifullin, Bulat F.,Kataev, Vladimir E.,Lyubina, Anna P.,Man’kova, Maria A.,Saifina, Liliya F.,Semenov, Vyacheslav E.,Shulaeva, Marina M.,Slita, Alexander V.,Volobueva, Alexandrina S.,Voloshina, Alexandra D.,Yesaulkova, Iana L.,Zarubaev, Vladimir V.
, (2021/07/06)
A series of 1,2,3-triazolyl nucleoside a...
The First Analog of Pyrimidine Nucleosides with Two Nucleobases and Two d-Ribofuranose Residues
Andreeva,Saifina,Belenok,Semenov,Kataev
, p. 292 - 296 (2021/03/26)
Abstract: The reaction of 1,5-bis[1-(pro...
A Synthesis Strategy for the Production of a Macrolactone of Gulmirecin A via a Ni(0)-Mediated Reductive Cyclization Reaction
Ichikawa, Satoshi,Katsuyama, Akira,Kitahata, Shun
supporting information, (2020/03/30)
A synthesis strategy for the production ...
13035-61-5 Process route
-
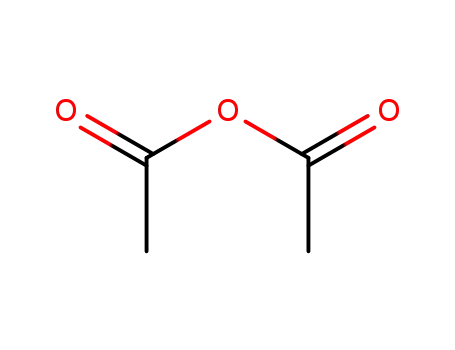
- 108-24-7
acetic anhydride

-
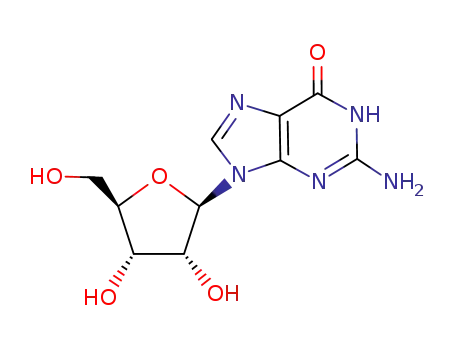
- 118-00-3
G

-
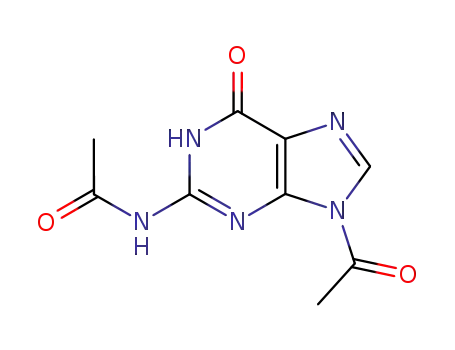
- 3056-33-5
2,9-diacetylguanine

-
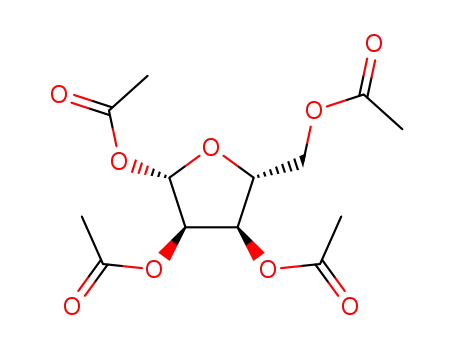
- 13035-61-5
1,2,3,5-tetraacetylribose
| Conditions | Yield |
|---|---|
|
acetic anhydride; G; at 136 ℃; for 1h;
With trifluoroacetic acid; at 60 - 100 ℃;
|
86% 96% |
-
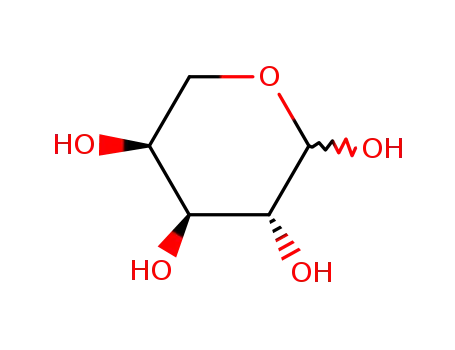
- 87-72-9,608-45-7,608-46-8,608-47-9,2460-44-8,6748-95-4,6763-34-4,7261-26-9,7283-06-9,7283-07-0,7296-55-1,7296-56-2,7296-58-4,7296-59-5,7296-60-8,7296-61-9,7296-62-0,7322-30-7,10257-31-5,10257-32-6,10257-33-7,10257-34-8,10257-35-9,19982-83-3,20242-88-0,28697-53-2,36562-42-2,41546-41-2,89299-64-9,107655-34-5,115794-06-4,115794-07-5,130550-15-1,130606-21-2
L-(+)-arabinose

-

- 108-24-7
acetic anhydride

-
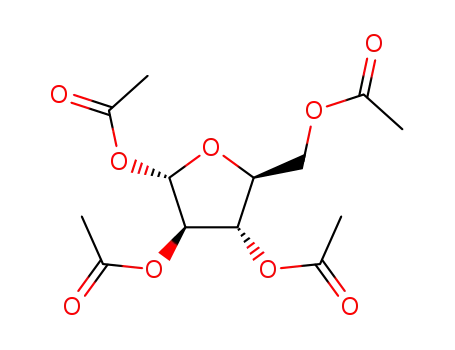
- 13035-61-5,23094-61-3,28708-32-9,39727-26-9,42927-46-8,43225-70-3,50730-26-2,56272-01-6,61248-15-5,61826-42-4,61849-90-9,69855-18-1,69855-19-2,79120-80-2,79120-81-3,86782-36-7,86782-94-7,105453-36-9,144490-03-9
1,2,3,6-tetra-O-acetyl-5-deoxy-α-L-arabino-hexofuranose

-
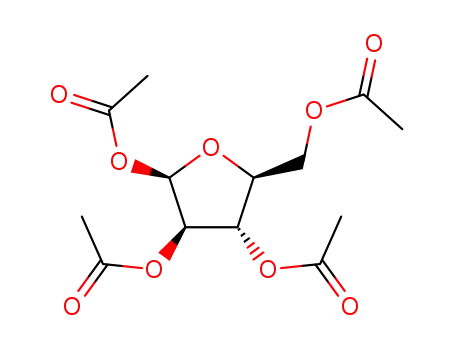
- 13035-61-5,23094-61-3,28708-32-9,39727-26-9,42927-46-8,43225-70-3,50730-26-2,56272-01-6,61248-15-5,61826-42-4,61849-90-9,69855-18-1,69855-19-2,79120-80-2,79120-81-3,86782-36-7,86782-94-7,105453-36-9,144490-03-9
1,2,3,6-tetra-O-acetyl-5-deoxy-β-L-arabino-hexofuranose

-
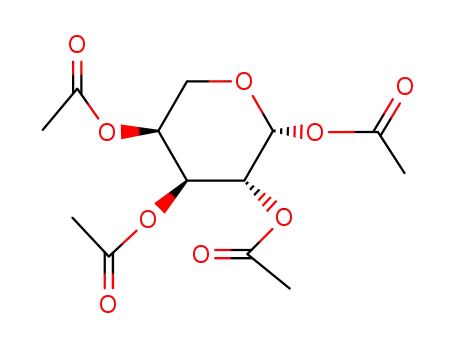
- 4258-00-8
1,2,3,4-tetra-O-acetyl-β-L-arabinopyranose

-
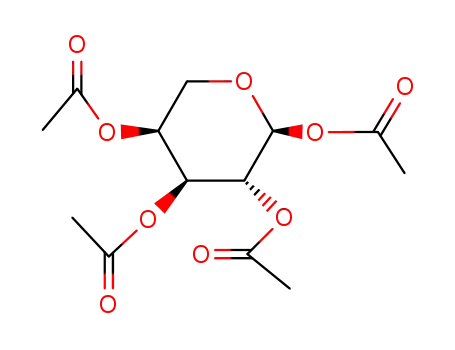
- 1233-03-0,2595-11-1,4026-34-0,4049-33-6,4049-34-7,4257-95-8,4257-98-1,4258-00-8,4627-30-9,17080-99-8,19186-37-9,25227-11-6,25243-38-3,62446-93-9,62929-49-1,67226-03-3,78087-60-2,78088-17-2,82890-16-2,86782-34-5,86782-35-6,92218-63-8,99880-95-2,108646-05-5,115939-79-2,123163-97-3,142130-89-0
1,2,3,4-tetra-O-acetyl-α-L-arabinopyranose
| Conditions | Yield |
|---|---|
|
With pyridine; at 0 ℃; Yield given. Yields of byproduct given;
|
|
|
With H-Beta zeolite; for 2h; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; Ambient temperature;
|
|
|
With sodium acetate; Yield given. Yields of byproduct given;
|
|
|
With pyridine; N,N-dimethyl-formamide; at 120 ℃; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With sodium acetate; Product distribution; other reagent, other temperature;
|
|
|
With pyridine; at 25 ℃; for 12h; Reagent/catalyst; Overall yield = 100 %; Overall yield = 11.11 g; stereoselective reaction; Inert atmosphere;
|
20 % de 20 % de |
13035-61-5 Upstream products
-
94482-37-8
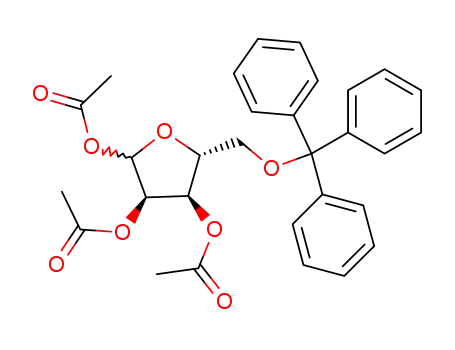
5-O-triphenylmethyl-D-ribofuranose-1,2,3-triacetate
-
506-96-7
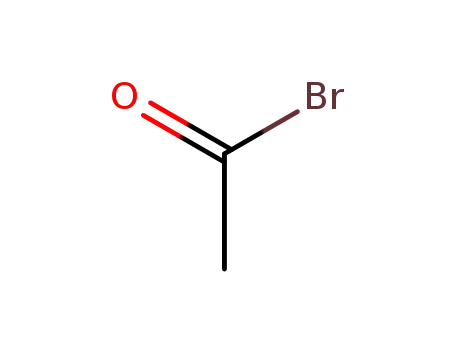
Acetyl bromide
-
108-24-7

acetic anhydride
-
110-86-1
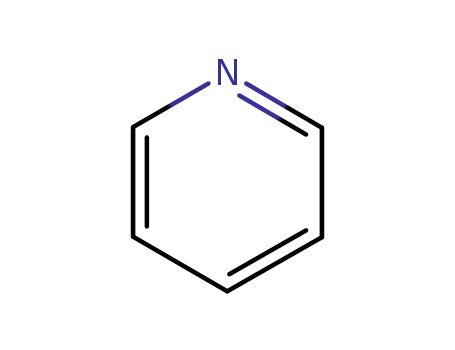
pyridine
13035-61-5 Downstream products
-
2140-61-6
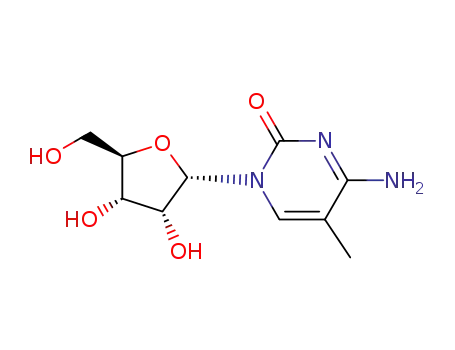
4-amino-5-methyl-1-α-D-ribofuranosyl-1H-pyrimidin-2-one
-
86480-36-6
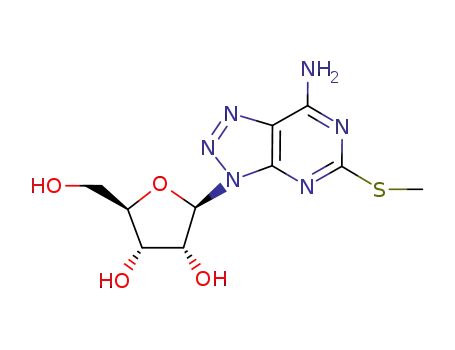
3-β-D-ribofuranosyl-5-(methylthio)-3H-1,2,3-triazolo<4,5-d>pyrimidin-7-amine
-
86480-42-4
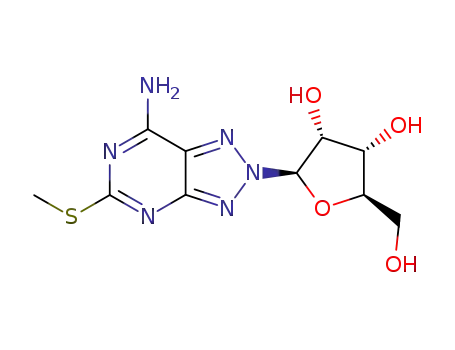
(2R,3R,4S,5R)-2-(7-Amino-5-methylsulfanyl-[1,2,3]triazolo[4,5-d]pyrimidin-2-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol
-
86480-43-5
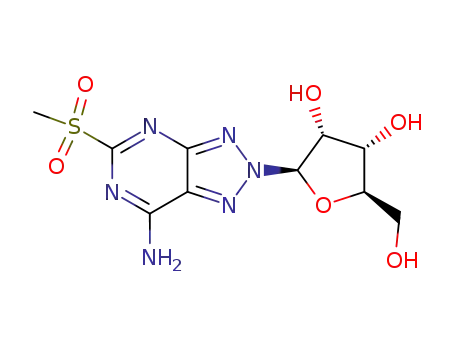
(2R,3R,4S,5R)-2-(7-Amino-5-methanesulfonyl-[1,2,3]triazolo[4,5-d]pyrimidin-2-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol
Relevant Products
-
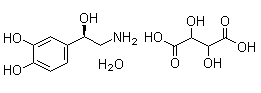
Epinephrine bitartrate
CAS:51-42-3
-
Norepinephrine Bitartrate
CAS:69815-49-2
-
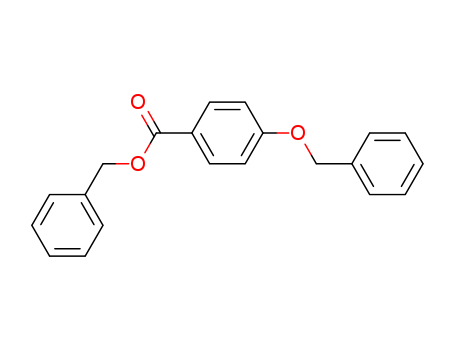
BENZYL 4-BENZYLOXYBENZOATE
CAS:56442-22-9