158798-73-3
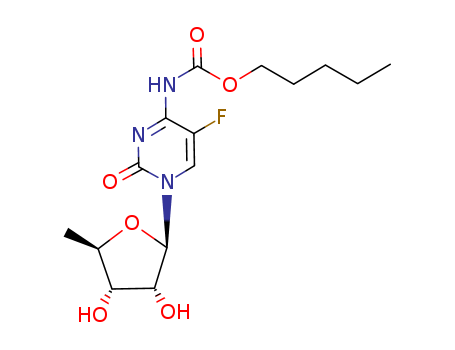
- Product Name:Capecitabine
- Molecular Formula:C15H22FN3O6
- Purity:99%
- Molecular Weight:359.355
Product Details;
CasNo: 158798-73-3
Molecular Formula: C15H22FN3O6
Reliable Quality Reputable Manufacturer Supply Capecitabine 158798-73-3 with Efficient Delivery
- Molecular Formula:C15H22FN3O6
- Molecular Weight:359.355
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.7
- Boiling Point:437.874°C at 760 mmHg
- Flash Point:218.619°C
- PSA:122.91000
- Density:1.49 g/cm3
- LogP:0.83320
Capecitabine(Cas 158798-73-3) Usage
|
Originator |
Xeloda,Hoffmann - La Roche Inc.,USA |
|
Therapeutic Function |
Antitumor |
InChI:InChI=1/C9H12FN3O4/c1-3-5(14)6(15)8(17-3)13-2-4(10)7(11)12-9(13)16/h2-3,5-6,8,14-15H,1H3,(H2,11,12,16)/t3-,5-,6-,8-/m1/s1
158798-73-3 Relevant articles
Preparation of capecitabine intermediate
-
Paragraph 0025-0028, (2021/09/01)
The invention belongs to the field of me...
Capecitabine intermediate
-
Paragraph 0043; 0107; 0110-0111, (2020/07/24)
The invention belongs to the field of me...
Cytidine derivative and method for preparing capecitabine medicines through derivative
-
Paragraph 0065-0069, (2020/05/14)
The invention discloses a 5-deoxy-D-ribo...
Continuous-Flow Sequential Schotten-Baumann Carbamoylation and Acetate Hydrolysis in the Synthesis of Capecitabine
Miranda, Leandro S. De M.,De Souza, Rodrigo O. M. A.,Lea?, Raquel A. C.,Carneiro, Paula F.,Pedraza, Sergio F.,De Carvalho, Otavio V.,De Souza, Stefania P.,Neves, Rebeca V.
, p. 2516 - 2520 (2019/11/03)
Capecitabine is an important anticancer ...
158798-73-3 Process route
-
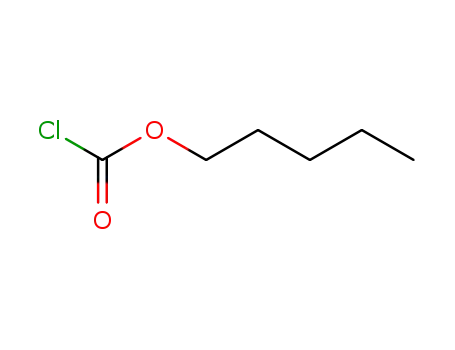
-
638-41-5
pentyl chloroformate

-
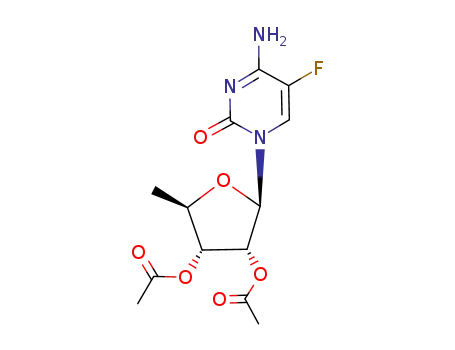
-
161599-46-8,2230479-30-6
(2R,3R,4R,5R)-2-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-5-methyl-tetrahydrofuran-3,4-diyl diacetate

-
-
154361-50-9,158798-73-3
capecitabine
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water; acetone;
at 30 ℃;
for 5h;
Green chemistry;
|
82% |
|
pentyl chloroformate; (2R,3R,4R,5R)-2-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-5-methyl-tetrahydrofuran-3,4-diyl diacetate;
With
pyridine;
In
dichloromethane;
at 0 ℃;
for 1h;
With
sodium hydroxide;
In
ethanol; water;
at -5 - 0 ℃;
for 0.233333h;
|
80.2% |
|
Multi-step reaction with 2 steps
1: dichloromethane
2: sodium hydroxide / methanol
With
sodium hydroxide;
In
methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: pyridine / dichloromethane / 1 h / 0 °C
2: sodium methylate / methanol / 1 h / 20 °C
With
pyridine; sodium methylate;
In
methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: potassium phosphate / isopropyl alcohol; dichloromethane / 4 h / 0 - 25 °C / Inert atmosphere
2: sodium hydroxide / methanol; water / 1 h / -10 - 5 °C / Autoclave
With
potassium phosphate; sodium hydroxide;
In
methanol; dichloromethane; water; isopropyl alcohol;
|
-
-
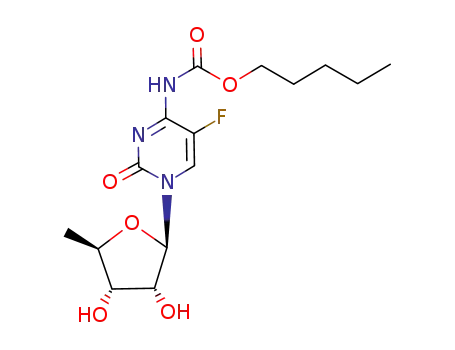
2'3'-O-isopropylidene-5'-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine

-

-
154361-50-9,158798-73-3
capecitabine
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid;
In
methanol; dichloromethane; water;
at 10 - 20 ℃;
for 3h;
Solvent;
|
89.4% |
158798-73-3 Upstream products
-
162204-20-8
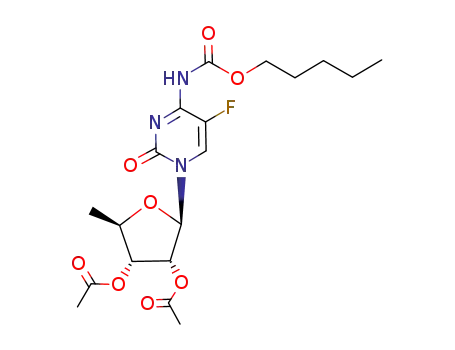
N1-(2',3'-di-O-acetyl-5'-deoxy-β-D-ribofuranosyl)-5-fluoro-N4-(pentyloxycarbonyl)cytosine
-
638-41-5

pentyl chloroformate
-
161599-46-8

(2R,3R,4R,5R)-2-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-5-methyl-tetrahydrofuran-3,4-diyl diacetate
-
27821-07-4
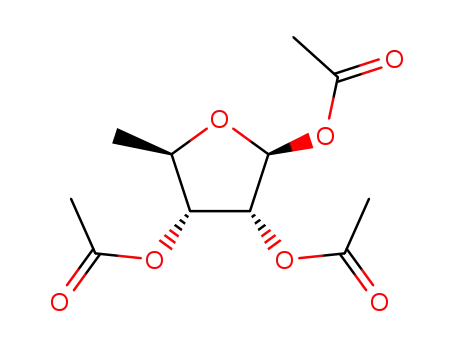
1,2,3-tri-O-acetyl-5-deoxy-β-D-ribofuranose
158798-73-3 Downstream products
-
66335-38-4
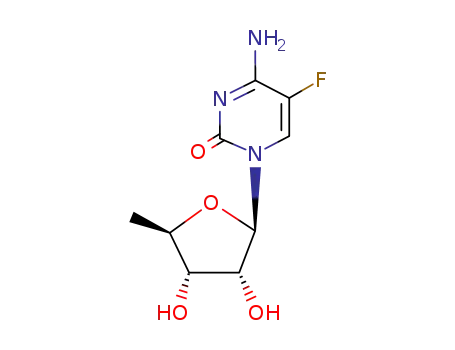
5'-deoxy-5-fluorocytidine
Relevant Products
-
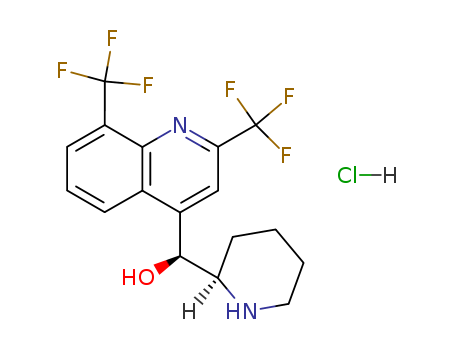
Mefloquine hydrochloride
CAS:51773-92-3
-
Fludarabine
CAS:21679-14-1
-
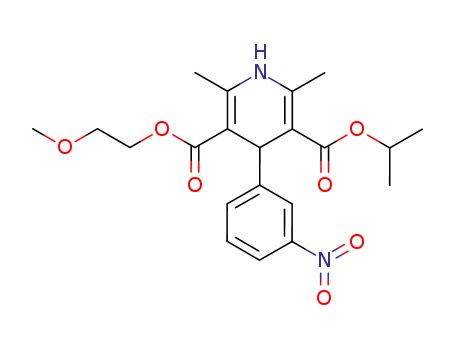
Nimodipine
CAS:66085-59-4