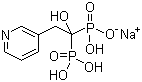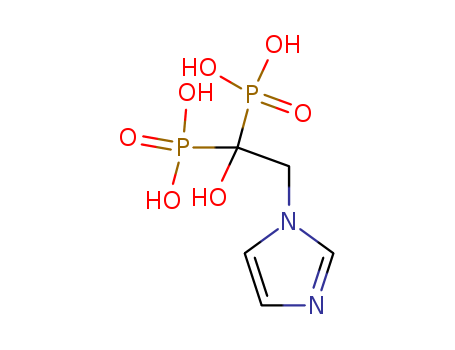
118072-93-8
- Product Name:Zoledronic acid
- Molecular Formula:C5H10N2O7P2
- Purity:99%
- Molecular Weight:316.055
Product Details;
CasNo: 118072-93-8
Molecular Formula: C5H10N2O7P2
Appearance: white crystalline powder
Wholesale Factory Supply Zoledronic acid 118072-93-8 with Low Price
- Molecular Formula:C5H10N2O7P2
- Molecular Weight:316.055
- Appearance/Colour:white crystalline powder
- Vapor Pressure:1.53E-24mmHg at 25°C
- Melting Point:193-2040 ºC
- Refractive Index:1.718
- Boiling Point:764 ºC at 760 mmHg
- PKA:1.41±0.10(Predicted)
- Flash Point:415.8 ºC
- PSA:172.73000
- Density:2.13 g/cm3
- LogP:-1.11540
Zoledronic acid(Cas 118072-93-8) Usage
|
Description |
Zoledronic acid is a white, crystalline powder that is available in vials for reconstitution for IV infusion over at least 15 minutes. It does not undergo metabolic transformation and does not inhibit CYP450 enzymes. Clearance of this agent is dependent on the patient's creatinine clearance, not on dose. Serum creatinine levels should be evaluated before every treatment. Zolendronic acid is contraindicated in patients with severe renal impairment. |
|
Chemical Properties |
White Solid |
|
Originator |
Zometa,Novartis Pharma,Switz |
|
Uses |
Bisphosphonate antiresorptive agent |
|
Definition |
ChEBI: An imidazole compound having a 2,2-bis(phosphono)-2-hydroxyethane-1-yl substituent at the 1-position. |
|
Manufacturing Process |
With stirring and under reflux, 8.6 g (0.053 mole) of imidazol-4-yl acetic acid hydrochloride, 7.1 ml of 85% phosphoric acid and 25 ml of chlorobenzene are heated to 100°C. Then 13.9 ml of phosphorus trichloride are added dropwise at 100°C, whereupon evolution of gas occurs. Over the course of 30 min a dense mass precipitates from the reaction mixture. The batch is heated for 3 hours to 100°C and the supernatant chlorobenzene is removed by decantation. With stirring and under reflux, the residual viscous mass is heated to the boil for 3 hours with 40 ml of 9 N hydrochloric acid. The batch is filtered hot with the addition of carbon and the filtrate is diluted with acetone, whereupon the crude 2-(imidazol-4-yl)-1-hydroxy-ethane-1,1- diphosphonic acid precipitates. This product is recrystallised from water. Melting point: 238-240°C (dec.). |
|
Brand name |
Zometa (Novartis). |
|
Therapeutic Function |
Bone calcium regulator |
|
Biological Functions |
Zoledronic acid, a bisphosphonate, was approved by the U.S. FDA in 2001 for the treatment of hypercalcemia of malignancy, a metabolic complication that can be life-threatening. Hypercalcemia of malignancy can occur in up to 50% of patients diagnosed with advanced breast cancer, multiple myeloma, and nonsmall cell lung cancer. This condition arises when chemical moieties produced by the tumor cause overstimulation of osteoclasts. When there is an increase in bone degradation, there is a concomitant release of calcium into the plasma. When serum concentrations of calcium rapidly elevate, the kidneys are unable to handle the overload, and hypercalcemia results. This can lead to dehydration, nausea, vomiting, fatigue, and confusion. Zoledronic acid effectively decreases plasma calcium concentrations via inhibition of bone resorption (inhibition of osteoclastic activity and induction of osteoclast apoptosis). It also prevents the increase in osteoclastic activity caused by tumor-based stimulatory factors. Additionally zoledronic acid has been approved by the U.S. FDA for the treatment of multiple myeloma and bone metastases associated with solid tumor–based cancers (e.g., prostrate and lung). This agent is currently in late-stage clinical trials for the treatment and prevention of osteoporosis and, if approved, will be formulated as a 5-mg, once-yearly IV infusion. |
|
Clinical Use |
Zoledronic acid is most commonly given to patients whose cancer is no longer responding to hormones, but it also may be given to prevent the bone thinning and weakening that results from hormonal treatments. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Other nephrotoxic drugs: use with caution as can enhance nephrotoxicity |
|
Metabolism |
Zoledronic acid is not metabolised and is excreted unchanged via the kidney. Over the first 24 hours, 39 ± 16% of the administered dose is recovered in the urine, while the remainder is principally bound to bone tissue. |
InChI:InChI=1/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14)
118072-93-8 Relevant articles
Pharmacological evaluation of imidazole-derived bisphosphonates on receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation and function
Lin, Jianguo,Peng, Ying,Liu, Qingzhu,Li, Ke,Lv, Gaochao,Seimbille, Yann,Huang, Gang,Gao, Feng,Qiu, Ling
, p. 121 - 133 (2021)
Bisphosphonates (BPs) have been commonly...
Silylation of hydroxyalkylidenediphosphonic acids as an effective method of their purification
Lebedev, A. V.,Sheludyakov, V. D.,Ustinova, O. L.,Lebedeva, A. B.
, (2012)
-
Zoledronic acid: Monoclinic and triclinic polymorphs from powder diffraction data
Chernyshev, Vladimir V.,Shkavrov, Sergey V.,Paseshnichenko, Ksenia A.,Puryaeva, Tamara P.,Velikodny, Yurii A.
, p. 263 - 266 (2013)
The crystal structures of the monoclinic...
“Greener” Synthesis of Zoledronic Acid from Imidazol-1-yl-acetic Acid and P-Reagents Using Diethyl Carbonate as the Solvent Component
Grün, Alajos,Keglevich, Gy?rgy,Szalai, Zsuzsanna
, p. 8 - 12 (2021/03/19)
The synthesis of a third generation dron...
The synthesis of hydroxymethylenebisphosphonic- (dronic-) and acyl-ethoxycarbonyl-methylphosphonate derivatives
Für, Csilla Sepsey,Grün, Alajos,Gulyás, Kinga V.,Keglevich, Gy?rgy,Szalai, Zsuzsanna,Zahár, Róbert
, (2021/12/23)
Recent results on the synthesis of subst...
Zoledronic acid intermediate compound
-
Paragraph 0056; 0059-0060; 0061; 0064-0065; 0066; 0069-0070, (2021/03/31)
The invention belongs to the technical f...
118072-93-8 Process route
-
-
C8H8N4O2*ClH

-
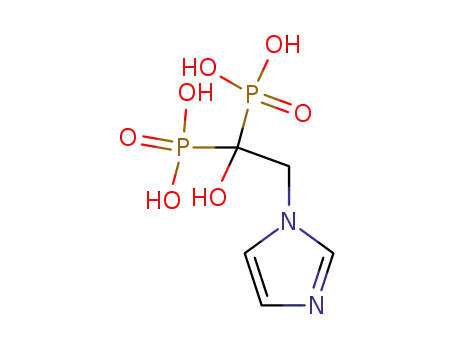
-
118072-93-8
1-hydroxy-2-(imidazol-1-yl)ethylidene-1,1-bisphosphonic acid
| Conditions | Yield |
|---|---|
|
C8H8N4O2*ClH;
With
phosphoric acid; phosphorus trichloride;
In
toluene;
at 90 ℃;
With
hydrogenchloride;
In
water; toluene;
at 20 ℃;
Reagent/catalyst;
Temperature;
Solvent;
Reflux;
|
85.27% |
-
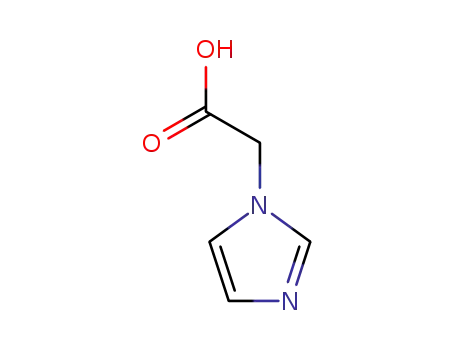
-
22884-10-2
imidazol-1-ylacetic acid

-

-
118072-93-8
1-hydroxy-2-(imidazol-1-yl)ethylidene-1,1-bisphosphonic acid
| Conditions | Yield |
|---|---|
|
imidazol-1-ylacetic acid;
With
phosphoric acid; phosphorus trichloride;
In
1,2-propylene cyclic carbonate; PEG 600 (polyethylene glycol);
at 40 - 60 ℃;
With
water;
In
1,2-propylene cyclic carbonate; PEG 600 (polyethylene glycol);
at 85 ℃;
for 18h;
Product distribution / selectivity;
|
99% |
|
imidazol-1-ylacetic acid;
With
phosphorous acid; phosphorus trichloride;
In
1,2-propylene cyclic carbonate; PEG 600;
at 40 - 60 ℃;
With
water;
In
1,2-propylene cyclic carbonate; PEG 600;
at 85 ℃;
for 18h;
Product distribution / selectivity;
|
99% |
|
With
sulfolane; phosphonic Acid; 1-butyl-3-methylimidazolium Tetrafluoroborate; phosphorus trichloride;
at 75 ℃;
for 3h;
Reagent/catalyst;
Green chemistry;
|
93% |
|
imidazol-1-ylacetic acid;
With
phosphorus trichloride;
at 50 - 55 ℃;
for 6h;
With
water;
at 70 - 80 ℃;
for 3h;
|
87.3% |
|
imidazol-1-ylacetic acid;
With
phosphorous acid; phosphorus trichloride;
In
N,N'- dimethylethyleneurea (DMEU);
at 40 - 60 ℃;
With
water;
In
N,N'- dimethylethyleneurea (DMEU);
at 80 - 100 ℃;
|
85.6% |
|
imidazol-1-ylacetic acid;
With
methanesulfonic acid; phosphorus trichloride;
at 55 ℃;
With
water;
at 8 - 70 ℃;
for 14 - 15h;
With
sodium hydroxide;
In
water;
at 30 - 40 ℃;
pH=0.25;
|
83% |
|
imidazol-1-ylacetic acid;
With
p-cresol; phosphoric acid; phosphorus trichloride;
for 8h;
Heating;
With
water;
Further stages.;
Heating;
|
80% |
|
imidazol-1-ylacetic acid;
With
phosphorous acid;
In
diphenylether;
at 70 ℃;
for 1h;
With
phosphorus trichloride;
In
diphenylether;
at 70 ℃;
for 6h;
With
water;
In
diphenylether; toluene;
at 25 ℃;
|
75% |
|
imidazol-1-ylacetic acid;
With
phosphorus trichloride;
In
methanesulfonic acid;
at 80 - 110 ℃;
for 8.5h;
With
sodium hydroxide;
for 24h;
pH=1.8;
Product distribution / selectivity;
|
74% |
|
imidazol-1-ylacetic acid;
With
phosphorous acid; trichlorophosphate;
In
silicon oil;
at 80 ℃;
for 17h;
With
water;
In
silicon oil;
at 80 - 100 ℃;
for 16h;
Product distribution / selectivity;
|
72% |
|
imidazol-1-ylacetic acid;
With
phosphorous acid;
In
sulfolane;
at 75 ℃;
for 0.5h;
With
phosphorus trichloride;
In
sulfolane;
at 35 - 67 ℃;
for 3h;
With
water;
In
sulfolane;
at 0 - 100 ℃;
for 8h;
|
70.7% |
|
imidazol-1-ylacetic acid;
With
phosphonic Acid; phosphorus trichloride;
In
sulfolane;
at 25 - 65 ℃;
for 0.0625h;
Microwave irradiation;
With
water;
In
sulfolane;
at 150 ℃;
for 0.166667h;
Microwave irradiation;
|
70% |
|
With
phosphoric acid; phosphorus trichloride;
In
chlorobenzene;
at 100 - 110 ℃;
for 3h;
|
67% |
|
With
phosphonic Acid; phosphorus trichloride;
at 75 ℃;
for 3h;
|
61% |
|
imidazol-1-ylacetic acid;
With
phosphorous acid; trichlorophosphate;
In
silicon oil;
at 80 ℃;
for 34h;
With
water;
In
toluene; silicon oil;
at 80 - 100 ℃;
for 16h;
Product distribution / selectivity;
|
59% |
|
imidazol-1-ylacetic acid;
With
phosphoric acid; phosphorus trichloride;
In
sulfolane;
at 54 - 88 ℃;
for 5.55 - 7.35h;
With
water;
In
sulfolane;
for 3 - 4h;
Product distribution / selectivity;
Heating / reflux;
|
53% |
|
imidazol-1-ylacetic acid;
With
phosphorus trichloride;
In
methanesulfonic acid;
at 80 ℃;
for 3h;
With
water;
In
methanesulfonic acid;
at 26 - 110 ℃;
for 5h;
With
sodium hydroxide;
In
water;
for 5h;
pH=1.8;
|
53% |
|
imidazol-1-ylacetic acid;
With
phosphorous acid; trichlorophosphate;
In
silicon oil;
at 80 ℃;
for 26h;
With
water;
In
ethanol; silicon oil;
at 0 - 97 ℃;
for 43.5h;
Product distribution / selectivity;
|
50% |
|
imidazol-1-ylacetic acid;
With
phosphonic Acid; phosphorus trichloride;
In
methanesulfonic acid;
at 75 ℃;
for 3h;
With
water;
Reagent/catalyst;
|
50% |
|
imidazol-1-ylacetic acid;
With
hydrogenchloride; phosphorus trichloride;
In
water;
at 0 - 5 ℃;
for 2.5h;
With
water;
for 6h;
Product distribution / selectivity;
Reflux;
|
49% |
|
imidazol-1-ylacetic acid;
With
hydrogenchloride; phosphorus trichloride;
In
water;
at 0 - 80 ℃;
for 2.5h;
With
water;
for 6h;
Product distribution / selectivity;
Heating;
|
49% |
|
With
phosphoric acid; phosphorus trichloride;
In
diethylene glycol dimethyl ether; water;
at 50 - 100 ℃;
for 8 - 11h;
Product distribution / selectivity;
Heating / reflux;
|
28% |
|
With
phosphoric acid; phosphorus trichloride;
In
water; PEG 400;
at 70 - 85 ℃;
for 11h;
Product distribution / selectivity;
Heating / reflux;
|
7% |
|
imidazol-1-ylacetic acid;
With
pyridine; hydrogenchloride; phosphorus trichloride;
at 95 ℃;
With
hydrogenchloride;
at 135 ℃;
|
|
|
imidazol-1-ylacetic acid;
With
pyridine; hydrogenchloride; phosphorus trichloride;
With
hydrogenchloride;
Heating;
|
|
|
imidazol-1-ylacetic acid;
With
phosphonic Acid;
In
sulfolane;
at 75 ℃;
for 0.5h;
With
phosphorus trichloride;
In
sulfolane;
at 35 - 67 ℃;
for 3h;
With
water;
at 0 - 100 ℃;
for 4h;
|
|
|
imidazol-1-ylacetic acid;
With
phosphorous acid;
In
1,2-dimethoxyethane;
at 75 ℃;
for 0.5h;
With
phosphorus trichloride;
In
1,2-dimethoxyethane;
at 35 - 67 ℃;
for 3h;
With
water;
In
1,2-dimethoxyethane;
at 0 - 100 ℃;
for 8h;
|
|
|
imidazol-1-ylacetic acid;
With
phosphorous acid;
In
octane;
at 90 - 95 ℃;
With
phosphorus trichloride;
In
octane;
at 90 - 95 ℃;
With
water;
In
octane;
at 90 - 95 ℃;
Product distribution / selectivity;
|
|
|
imidazol-1-ylacetic acid;
With
phosphorous acid;
In
1,4-dioxane;
at 90 - 95 ℃;
With
phosphorus trichloride;
In
1,4-dioxane;
at 90 - 95 ℃;
With
water;
In
1,4-dioxane;
at 90 - 95 ℃;
Product distribution / selectivity;
|
|
|
imidazol-1-ylacetic acid;
With
phosphorous acid; phosphorus trichloride;
at 60 - 80 ℃;
for 6h;
With
hydrogenchloride; water;
at 75 - 80 ℃;
for 12.5h;
Product distribution / selectivity;
|
|
|
imidazol-1-ylacetic acid;
With
phosphorous acid; trichlorophosphate;
at 60 - 80 ℃;
for 5h;
With
hydrogenchloride; water;
at 90 ℃;
for 12.5h;
Product distribution / selectivity;
|
|
|
imidazol-1-ylacetic acid;
With
phosphorous acid; phosphorus trichloride;
In
octane;
at 90 - 95 ℃;
With
water;
In
octane;
at 90 - 95 ℃;
Product distribution / selectivity;
|
|
|
Multi-step reaction with 2 steps
1.1: methanesulfonic acid; bis(trichloromethyl) carbonate / 4 h / 80 °C
2.1: methanesulfonic acid; phosphorus trichloride / 16 h / 26 - 80 °C
2.2: 5 h / 26 - 110 °C
2.3: 12 h / pH 1.8
With
methanesulfonic acid; bis(trichloromethyl) carbonate; phosphorus trichloride;
|
|
|
imidazol-1-ylacetic acid;
With
phosphoric acid; silica gel; phosphorus trichloride;
In
neat (no solvent);
at 80 ℃;
for 0.05h;
Microwave irradiation;
With
water;
In
neat (no solvent);
at 100 ℃;
for 0.05h;
Microwave irradiation;
|
|
|
imidazol-1-ylacetic acid;
With
phosphoric acid;
In
water;
at 100 ℃;
for 0.5h;
With
phosphorus trichloride;
In
water;
at 65 - 100 ℃;
for 3h;
With
hydrogenchloride;
In
water;
for 5h;
Reflux;
|
|
|
With
phosphoric acid; water; phosphorus trichloride;
In
chlorobenzene;
|
118072-93-8 Upstream products
-
22884-10-2

imidazol-1-ylacetic acid
-
87266-37-3
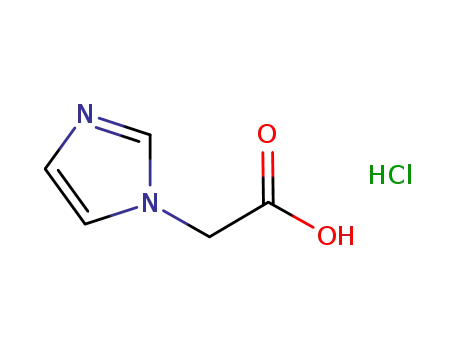
2-(1-imidazolyl)acetic acid hydrochloride
-
160975-66-6
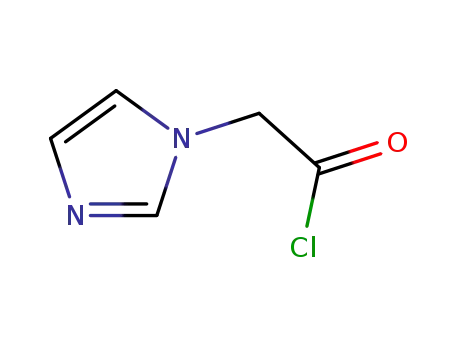
imidazol-1-yl-acetyl chloride
-
1392687-52-3
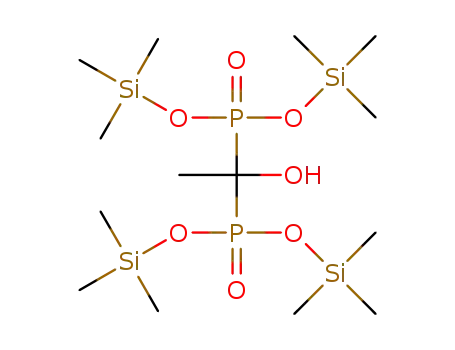
tetrakis(O-trimethylsilyl)hydroxyethylidenediphosphonic acid
118072-93-8 Downstream products
-
904894-54-8
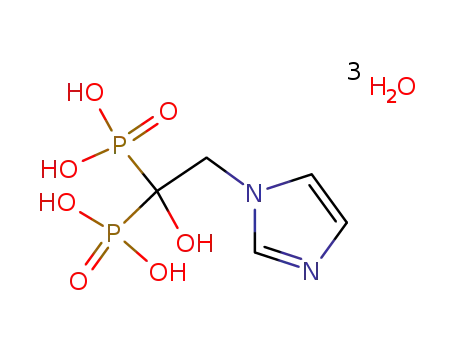
zoledronic acid trihydrate
-
165800-06-6
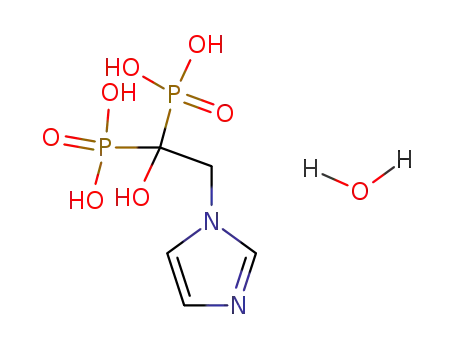
zoledronic acid monohydrate
-
1296134-54-7
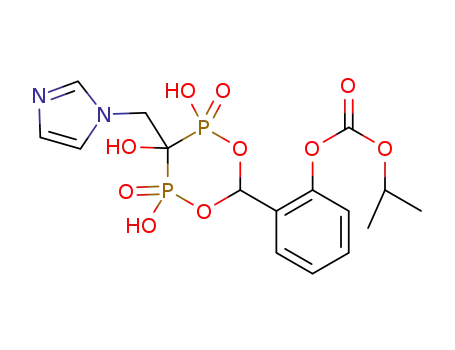
Carbonic acid isopropyl ester 2-(2,3,4-trihydroxy-3-imidazol-1-ylmethyl-2,4-dioxo-2λ5,4λ5-[1,5,2,4]dioxadiphosphinan-6-yl)-phenyl ester
Relevant Products
-
Sodium risedronate
CAS:115436-72-1
-
chlorophyllin copper complex sodium salt
CAS:65963-40-8