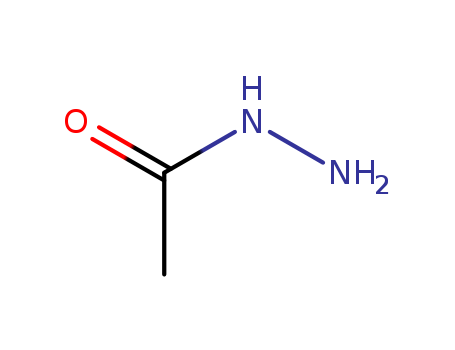
1068-57-1
- Product Name:Acethydrazide
- Molecular Formula:C2H6N2O
- Purity:99%
- Molecular Weight:74.0824
Product Details;
CasNo: 1068-57-1
Molecular Formula: C2H6N2O
Appearance: white solid
Factory Supply High Purity 1068-57-1 with Reasonable Price, Buy Quality Acethydrazide
- Molecular Formula:C2H6N2O
- Molecular Weight:74.0824
- Appearance/Colour:white solid
- Vapor Pressure:1.07mmHg at 25°C
- Melting Point:58-68 °C(lit.)
- Refractive Index:1.453
- Boiling Point:246 °C at 760 mmHg
- PKA:13.46±0.18(Predicted)
- Flash Point:102.6 °C
- PSA:55.12000
- Density:1.041 g/cm3
- LogP:0.08740
Acethydrazide(Cas 1068-57-1) Usage
|
Description |
Hydrazine, anhydrous appears as a colorless, fuming oily liquid with an ammonia-like odor. Flash point 99°F. Explodes during distillation if traces of air are present. Toxic by inhalation and by skin absorption. Corrosive to tissue. Produces toxic oxides of nitrogen during combustion. |
|
Chemical Properties |
White moist crystals and chunks |
|
Uses |
Acetohydrazide is a metabolite of Isoniazid (I821450) an antibiotic for treatment of Mycobacterium tuberculosis, inhibits mycolic acid biosynthesis. |
|
Definition |
ChEBI: A carbohydrazide that is hydrazine in which one of the hydrogens is replaced by an acetyl group. |
|
Safety Profile |
Poison by ingestion, subcutaneous, and intraperitoneal routes. Mutation data reported. Exposure can cause hemolysis and liver damage. See also PHENYLHYDRAZINE. When heated to decomposition it emits toxic fumes of NOX,. |
|
Purification Methods |
Acetic hydrazide crystallises as needles from EtOH. It reduces NH3/AgNO3. [Beilstein 2 H 191, 2 IV 435.] |
InChI:InChI=1/C7H5ClF3N/c1-4-2-5(7(9,10)11)3-6(8)12-4/h2-3H,1H3
1068-57-1 Relevant articles
Asymmetric Synthesis and Antitumor Activity of Spiro-Oxadiazole Derivatives from 1,4:3,6-Dianhydro-D-fructose
Xu, Wenke,Ge, Yongxun,Hou, Yu,Liu, Yingju,Hua, Yingchun,Han, Weiwei,Qin, Zhiyan,Liu, Fengwu
, p. 1437 - 144 (2017)
A series of spiro-oxadiazoles were synth...
Pentanuclear [2.2] spirocyclic lanthanide(iii) complexes: slow magnetic relaxation of the DyIII analogue
Biswas, Sourav,Das, Sourav,Van Leusen, Jan,K?gerler, Paul,Chandrasekhar, Vadapalli
, p. 19282 - 19293 (2015)
The reaction of LnCl3·6H2O (Ln = Dy3+, T...
Facile synthesis of new 10-substituted-5H-naphtho[1,2-e][1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-5-ones
Khalafy, Jabbar,Mohammadlou, Mahsa,Mahmoody, Miri,Salami, Fatemeh,Poursattar Marjani, Ahmad
, p. 1528 - 1530 (2015)
The reaction of 2-bromo-1,4-naphthoquino...
Synthesis, crystal structure and biological activity of 4-Cyclopropyl-3-[(3-fluorobenzyl)thio]-5-methyl-4H-1,2,4-triazole Monohydrate
Ke, Wei,Sun, Na-Bo,Wu, Hong-Ke
, p. 8723 - 8726 (2013)
The compound 4-cyclopropyl-3-[(3-fluorob...
Consecutive hydrazino-Ugi-azide reactions: Synthesis of acylhydrazines bearing 1,5-disubstituted tetrazoles
De Fátima Barreto, Angélica S.,Dos Santos, Veronica Alves,Zandrade, Carlos Kleber
, p. 2596 - 2602 (2017)
Isocyanide-based multicomponent reaction...
-
Cavier,R.,Rips,R.
, p. 706 - 708 (1965)
-
Novel route for the transformation of a pyrimidine ring using hydrazides
Danagulyan,Tadevosyan,Tamazyan,Panosyan
, p. 233 - 245 (2006)
It has been shown from X-ray structural ...
-
Fastrez
, p. 7004 (1977)
-
Acid hydrolysis of 1,6-dihydro-4-amino-3-methyl-6-phenyl-1,2,4-triazin- 5(4H)-one (1,6-dihydrometamitron)
Ludvik,Jirkovsky,Urban,Zuman
, p. 3879 - 3885 (1999)
Metamitron (1) does not undergo hydrolys...
Iminoboronate Formation Leads to Fast and Reversible Conjugation Chemistry of α-Nucleophiles at Neutral pH
Bandyopadhyay, Anupam,Gao, Jianmin
, p. 14748 - 14752 (2015)
Bioorthogonal reactions that are fast an...
Catalytic conversion of glycerol to allyl alcohol; Effect of a sacrificial reductant on the product yield
Sanchez, Gizelle,Friggieri, Jarrod,Adesina, Adesoji A.,Dlugogorski, Bogdan Z.,Kennedy, Eric M.,Stockenhuber, Michael
, p. 3090 - 3098 (2014)
A continuous process for the conversion ...
Dual signaling of hydrazine by selective deprotection of dichlorofluorescein and resorufin acetates
Choi, Myung Gil,Moon, Jung Ok,Bae, Jihee,Lee, Jung Woo,Chang, Suk-Kyu
, p. 2961 - 2965 (2013)
The highly selective chemosignaling beha...
Synthesis and in vitro leishmanicidal activity of some hydrazides and their analogues
Khan, Khalid Mohammad,Rasheed, Maimona,Ullah, Zia,Hayat, Safdar,Kaukab, Farhana,Choudhary, M. Iqbal,Ur-Rahman, Atta,Perveen, Shahnaz
, p. 1381 - 1387 (2003)
Twenty-one hydrazides were synthesized b...
An efficient CO2 fixation reaction with epoxides catalysed by: In situ formed blue vanadium catalyst from dioxovanadium(+5) complex: Moisture enhanced and atmospheric oxygen retarded catalytic activity
Borah, Rakhimoni,Deori, Naranarayan,Brahma, Sanfaori
, p. 2547 - 2554 (2020)
Two new dioxovanadium(+5) complexes, one...
Bioisosteric approach to elucidation of binding of the acetate group of a moth sex pheromone component to its receptor
Gustavsson, Anna-Lena,Tuvesson, Malena,Larsson, Mattias C.,Wenqi, Wu,Hansson, Bill S.,Liljefors, Tommy
, p. 2755 - 2776 (1997)
A number of analogs of (Z)-5-decenyl ace...
Tuning the exchange dynamics of boronic acid hydrazones and oximes with pH and redox control
Han, Gun Su,Domaille, Dylan W.
, p. 4986 - 4991 (2021)
Dynamic bonds continually form and disso...
Catalytic oxygen atom transfer promoted by tethered Mo(VI) dioxido complexes onto silica-coated magnetic nanoparticles
Colaiezzi, Roberta,Crucianelli, Marcello,Di Giuseppe, Andrea,Ferella, Francesco,Lazzarini, Andrea,Paolucci, Valentina
, (2021/11/30)
The preparation of three novel active an...
Synthesis of novel seco-acyclo-N-diazolyl-thione nucleosides analogous derived from acetic acid: characterization, complex formation with ions Pb(II), Hg(II) and antibacterial activity
Chehrouri, Manel,Othman, Adil A.
, (2021/08/09)
Three diazoles namely 5-methyl-1,3,4-oxa...
Design, Synthesis, and Study of the Insecticidal Activity of Novel Steroidal 1,3,4-Oxadiazoles
Bai, Hangyu,Jiang, Weiqi,Li, Qi,Li, Tian,Ma, Shichuang,Shi, Baojun,Wu, Wenjun
, p. 11572 - 11581 (2021/10/12)
A series of novel steroidal derivatives ...
N-Amino-1,8-Naphthalimide is a Regenerated Protecting Group for Selective Synthesis of Mono-N-Substituted Hydrazines and Hydrazides
Manoj Kumar, Mesram,Venkataramana, Parikibanda,Yadagiri Swamy, Parikibanda,Chityala, Yadaiah
supporting information, p. 17713 - 17721 (2021/11/10)
A new route to synthesis of various mono...
1068-57-1 Process route
-
- 56-81-5,25618-55-7,64333-26-2,8013-25-0
glycerol

-
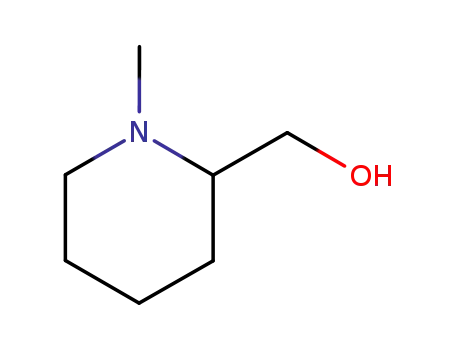
- 20845-34-5
(1-methyl-piperidin-2-yl)-methanol

-
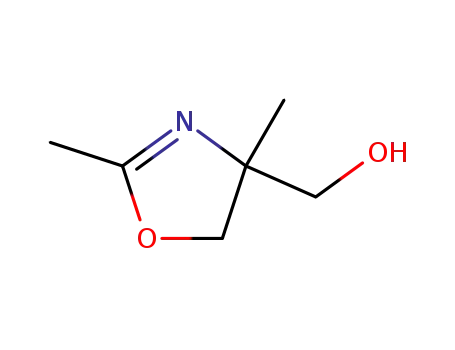
- 39986-37-3
2,4-dimethyl-2-oxazoline-4-methanol

-
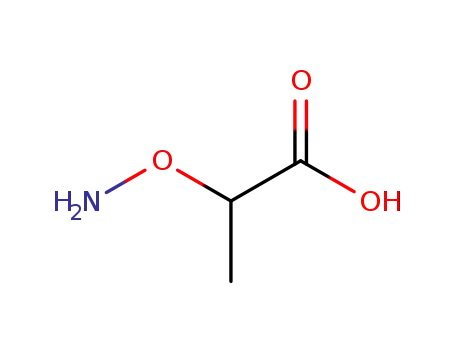
- 2786-22-3
2-(aminooxy)propanoic acid

-
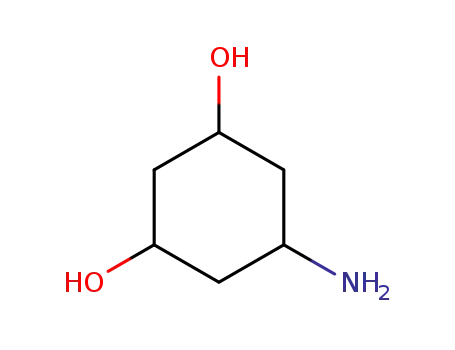
-
3,5-dihydroxycyclohexanamine

-
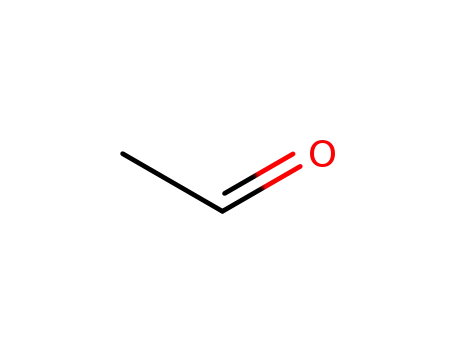
- 75-07-0,9002-91-9
acetaldehyde

-
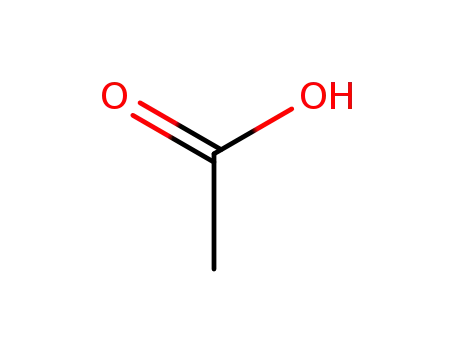
- 64-19-7,77671-22-8
acetic acid

-
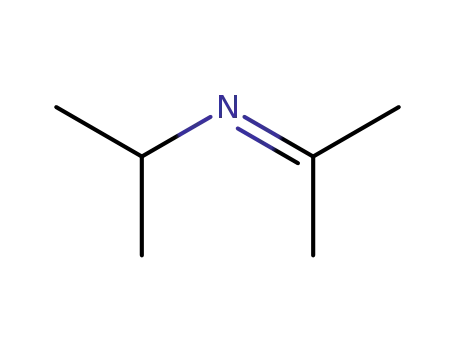
- 3332-08-9
2-isopropyliminopropane

-
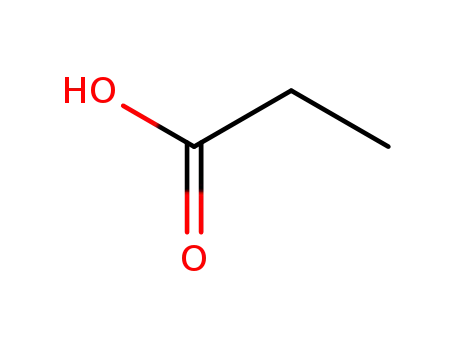
- 802294-64-0,79-09-4
propionic acid

-
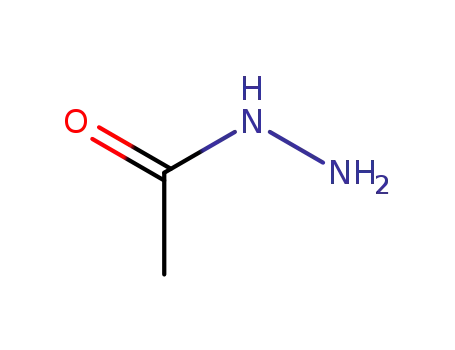
- 1068-57-1
acetic acid hydrazide

-
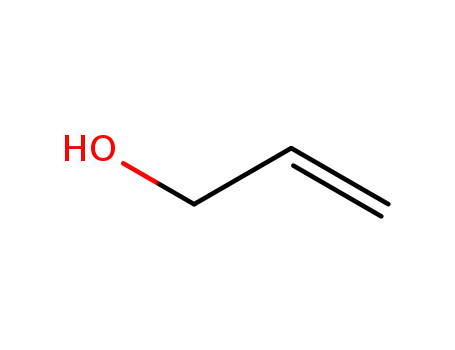
- 107-18-6
allyl alcohol

-
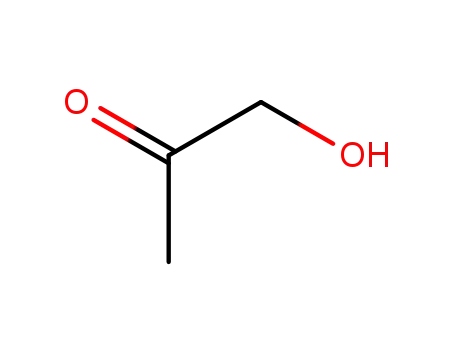
- 116-09-6
hydroxy-2-propanone

-
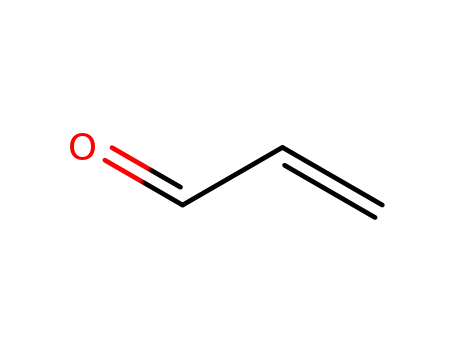
- 107-02-8,25068-14-8
acrolein

-
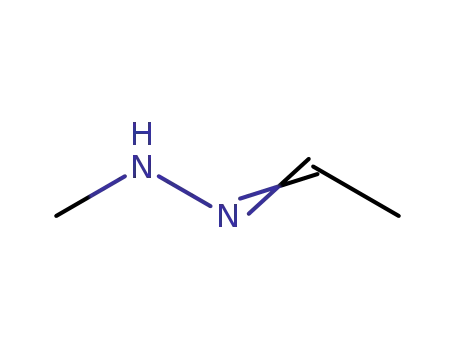
- 17167-73-6
acetaldehyde methylhydrazone

-
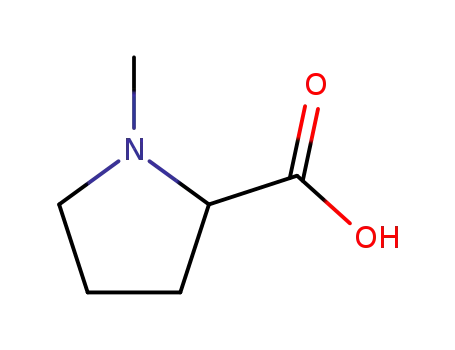
- 68078-09-1
N-methylproline
| Conditions | Yield |
|---|---|
|
With alumina-supported iron; ammonia; In water; at 340 ℃; Inert atmosphere;
|
11.3% 7.8% 5% 6.7% |
-
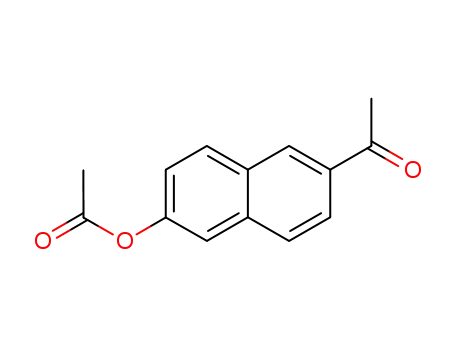
- 63256-69-9
6-acetyl-2-naphthyl acetate

-
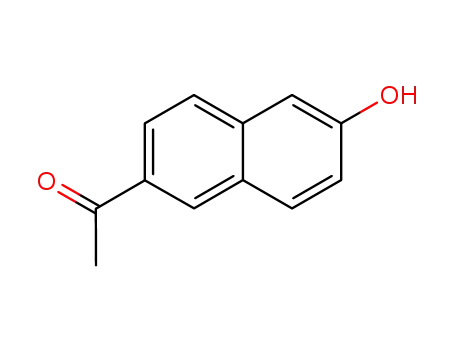
- 10441-41-5
1-(6-hydroxy-2-naphthyl)ethan-1-one

-

- 1068-57-1
acetic acid hydrazide
| Conditions | Yield |
|---|---|
|
With hydrazine; In water; at 30 ℃; pH=6.42; Further Variations:; pH-values; Kinetics;
|
1068-57-1 Upstream products
-
625-77-4
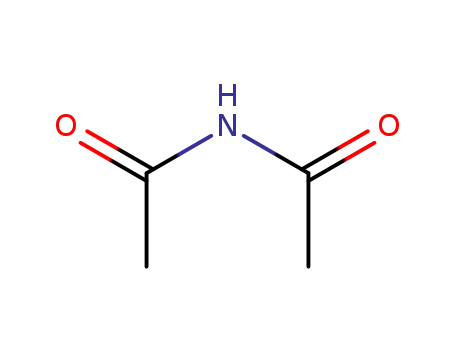
N-acetylacetamide
-
7335-65-1
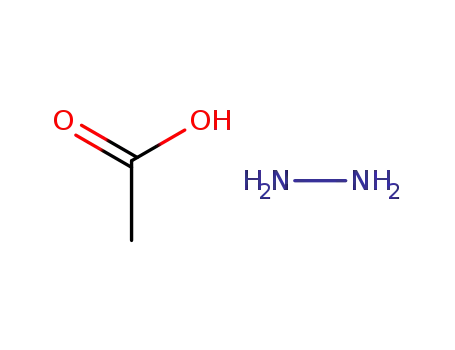
hydrazinium monoacetate
-
108-24-7
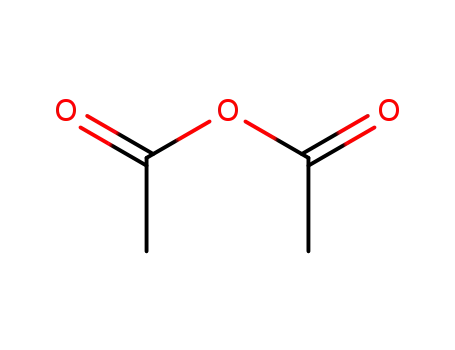
acetic anhydride
-
141-78-6
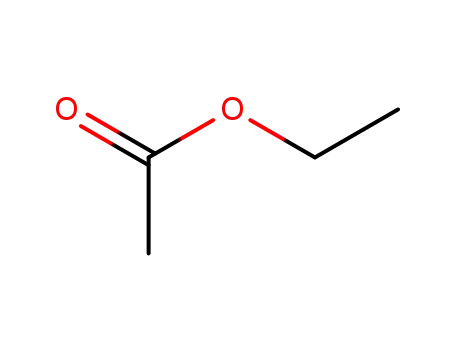
ethyl acetate
1068-57-1 Downstream products
-
118872-35-8
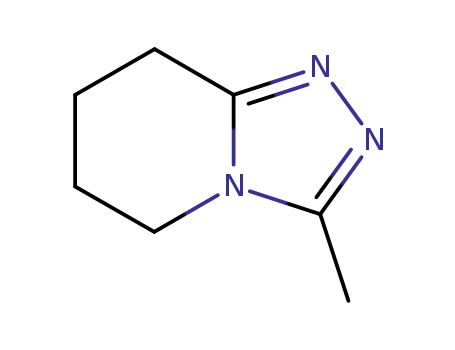
3-methyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyridine
-
2799-91-9
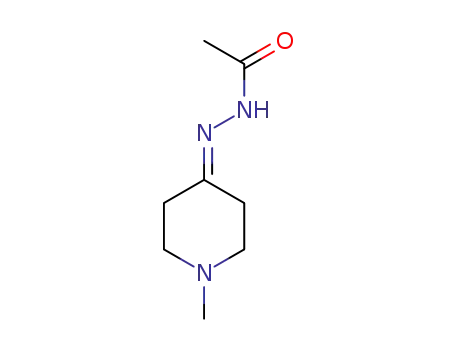
acetic acid-(1-methyl-[4]piperidylidenehydrazide)
-
28766-50-9
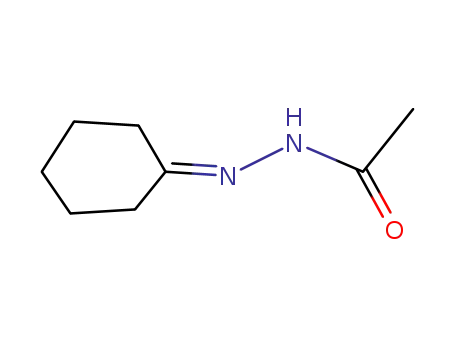
1-acetyl-2-(1-cyclohexylidene)hydrazine
-
3148-73-0
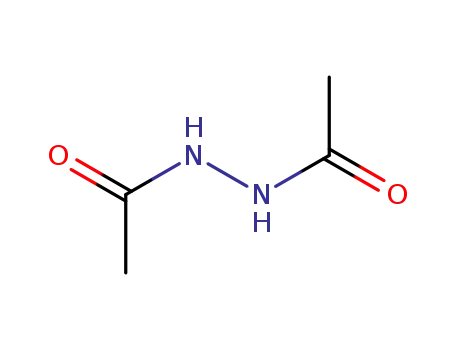
Diacetylhydrazin
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
3,5,6-Trichlorosalicylic acid
CAS:40932-60-3