75607-67-9
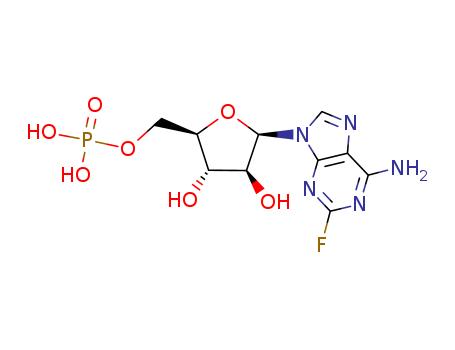
- Product Name:fludarabine phosphate
- Molecular Formula:C10H13FN5O7P
- Purity:99%
- Molecular Weight:365.215
Product Details;
CasNo: 75607-67-9
Molecular Formula: C10H13FN5O7P
Trustworthy Manufacturer Supply Best Quality fludarabine phosphate 75607-67-9 with Safe Shipping
- Molecular Formula:C10H13FN5O7P
- Molecular Weight:365.215
- Vapor Pressure:7.38E-32mmHg at 25°C
- Melting Point:203°C(dec.)(lit.)
- Refractive Index:1.878
- Boiling Point:864.2 °C at 760 mmHg
- PKA:1.86±0.10(Predicted)
- Flash Point:476.4 °C
- PSA:195.88000
- Density:2.39 g/cm3
- LogP:-1.14270
Fludarabine phosphate(Cas 75607-67-9) Usage
|
Description |
Fludarabine phosphate is an antimetabolite indicated for the treatment of B cell lymphocytic leukemia. It is reportedly effective in patients refractory to other therapies. Fludarabine phosphate acts by inhibiting primer RNA synthesis. Its side effects include bone marrow suppression, anemia, thrombocytopenia and neutropenia. |
|
Originator |
Southern Research Institute (U.S.A.) |
|
Uses |
anticonvulsant. Fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. This metabolite appears to act by inhibiting DNA polymerase alpha, ribonucleotide reductase and DNA primase, thus inhibiting DNA synthesis. The mechanism of action of this antimetabolite is not completely characterized and may be multi-faceted. Phase I studies in humans have demonstrated that fludarabine phosphate is rapidly converted to the active metabolite, 2-fluoro-ara-A, within minutes after intravenous infusion. Consequently, clinical pharmacology studies have focused on 2-fluoro-ara-A pharmacokinetics. After the five daily doses of 25 mg 2-fluoro-ara-AMP/m2 to cancer patients infused over 30 minutes, 2-fluoro-ara-A concentrations show a moderate accumulation. During a 5-day treatment schedule, 2-fluoro-ara-A plasma trough levels increased by a factor of about 2. The terminal half-life of 2-fluoro-ara-A was estimated as approximately 20 hours. In vitro, plasma protein binding of fludarabine ranged between 19% and 29%. |
|
Brand name |
Fludara (Berlex). |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antipsychotics: avoid concomitant use with clozapine, increased risk of agranulocytosis. Cytotoxics: increased pulmonary toxicity with pentostatin (unacceptably high incidence of fatalities); increases intracellular concentration of cytarabine. |
|
Metabolism |
Intravenous fludarabine phosphate is rapidly dephosphorylated to fludarabine which is taken up by lymphocytes and rephosphorylated via the enzyme deoxycytidine kinase to the active triphosphate nucleotide. Clearance of fludarabine from the plasma is triphasic; elimination is mostly via renal excretion: 40-60% of an intravenous dose is excreted in the urine. The pharmacokinetics of fludarabine show considerable inter-individual variation |
InChI:InChI=1/C10H13FN5O7P/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,14,15)(H2,19,20,21)/t3-,5-,6+,9-/m1/s1
75607-67-9 Relevant articles
Synthesis method of fludarabine phosphate
-
Paragraph 0041-0043; 0061-0065, (2020/08/22)
The invention provides a synthesis metho...
A 9 - β - D - arabinofuranosyl -2 - fluoro adenine -5 ' - phosphate ester preparation method (by machine translation)
-
Paragraph 0033; 0034; 0035; 0036; 0037; 0039; 0040; 0042, (2018/09/26)
The invention discloses a preparation me...
Fludarabine phosphate preparation method
-
Paragraph 0009, (2017/01/26)
The invention discloses a fludarabine ph...
Immobilized Drosophila melanogaster deoxyribonucleoside kinase (DmdNK) as a high performing biocatalyst for the synthesis of purine arabinonucleotides
Serra, Immacolata,Conti, Silvia,Piskur, Jure,Clausen, Anders R.,Munch-Petersen, Birgitte,Terreni, Marco,Ubiali, Daniela
, p. 563 - 570 (2014/05/20)
Fruit fly (Drosophila melanogaster) deox...
75607-67-9 Upstream products
-
146-78-1
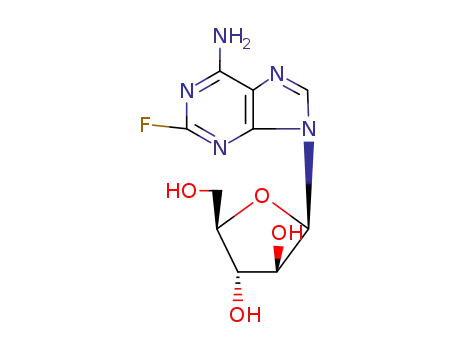
Fludarabine
-
512-56-1
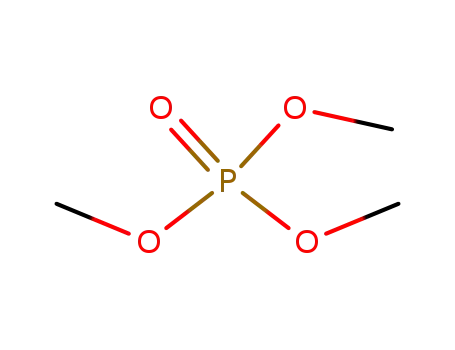
trimethyl phosphite
-
78-40-0
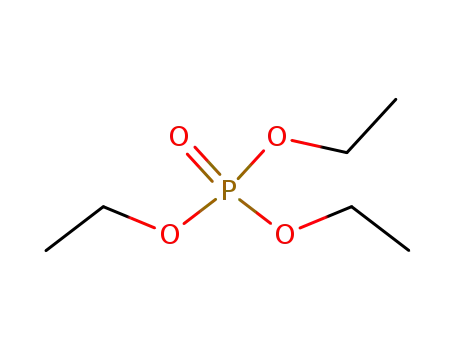
triethyl phosphate
-
7440-44-0
pyrographite
75607-67-9 Downstream products
-
146-78-1

Fludarabine
Relevant Products
-
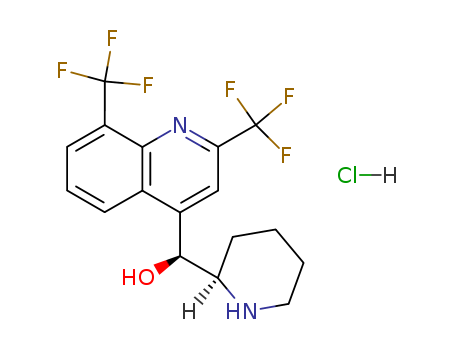
Mefloquine hydrochloride
CAS:51773-92-3
-
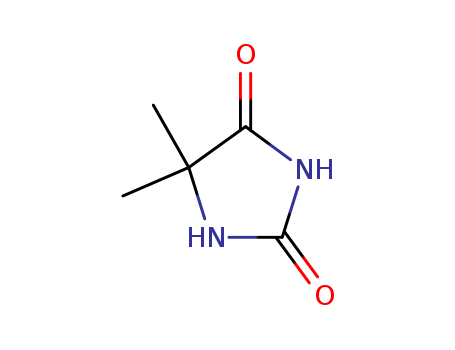
5,5-Dimethylhydantoin
CAS:77-71-4
-
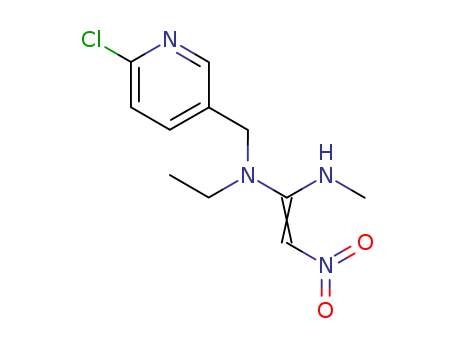
Nitenpyram
CAS:120738-89-8