68-94-0
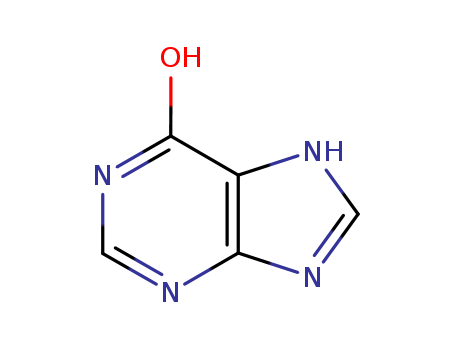
- Product Name:Hypoxanthine
- Molecular Formula:C5H4N4O
- Purity:99%
- Molecular Weight:136.113
Product Details;
CasNo: 68-94-0
Molecular Formula: C5H4N4O
Appearance: White to off-white powder
Trustworthy Manufacturer Supply Top Purity Hypoxanthine 68-94-0 with Cheapest Price
- Molecular Formula:C5H4N4O
- Molecular Weight:136.113
- Appearance/Colour:White to off-white powder
- Vapor Pressure:0.0148mmHg at 25°C
- Melting Point:250 °C
- Refractive Index:1.902
- Boiling Point:533.428 °C at 760 mmHg
- PKA:8.7(at 25℃)
- Flash Point:276.407 °C
- PSA:74.43000
- Density:1.892 g/cm3
- LogP:-0.35380
6-Hydroxypurine(Cas 68-94-0) Usage
|
Description |
Hypoxanthine is a naturally occurring purine derivative and intermediate in the synthesis of uric acid. It is elevated in the spinal fluid of patients with Lesch-Nyhan syndrome, a metabolic disorder whose symptoms include cerebral palsy, cognitive deficits, motor dysfunction, self-mutilation, and hyperuricemia. Injection of hypoxanthine (10 μM) increases succinate dehydrogenase and complex II activities and decreases cytochrome c oxidase activity, resulting in neuroenergetic impairment, ATP depletion, and cellular apoptosis in rat striatum. It is also used to induce hyperuricemia in mice for use in the development of hypouricemic agents. |
|
Chemical Properties |
White to off-white powder |
|
Uses |
A naturally occurring purine derivative. Pharmaceuticals, Intermediates & Fine Chemicals |
|
Definition |
ChEBI: A purine nucleobase that consists of purine bearing an oxo substituent at position 6. |
|
General Description |
Hypoxanthine (6-hydroxypurine), a purine derivative is a naturally occurring compound. It is the deaminated form of adenine and a breakdown product of adenosine monophosphate (AMP). |
|
Biochem/physiol Actions |
Hypoxanthine?is capable of stimulating cell death. It can also induce reactive oxygen species (ROS). It results in endothelial dysfunction via apoptosis, stimulated by oxidative stress. |
|
Safety Profile |
Moderately toxic by intraperitoneal route. An experimental teratogen. When heated to decomposition it emits toxic fumes of Nox |
|
Purification Methods |
Crystallise it from hot water and dry it at 105o. [Beilstein 26 II 252, 26 III/IV 2081.] |
InChI:InChI=1/C5H4N4O/c10-5-3-4(7-1-6-3)8-2-9-5/h1-3H,(H,6,7,8,9,10)
68-94-0 Relevant articles
The inhibitory effect of citrus flavonoids naringenin and hesperetin against purine nucleoside phosphorylase: Spectroscopic, atomic force microscopy and molecular modeling studies
Gong, Deming,Lv, Xingang,Ren, Er-Fang,Wang, Lang-Hong,Wang, Qilei
, (2020)
In this work, the inhibitory effect of t...
Ribocation Transition State Capture and Rebound in Human Purine Nucleoside Phosphorylase
Ghanem, Mahmoud,Murkin, Andrew S.,Schramm, Vern L.
, p. 971 - 979 (2009)
Purine nucleoside phosphorylase (PNP) ca...
Calcium-stimulated guanosine-inosine nucleosidase from yellow lupin (Lupinus luteus)
Szuwart, Maciej,Starzynska, Elzbieta,Pietrowska-Borek, Malgorzata,Guranowski, Andrzej
, p. 1476 - 1485 (2006)
Guanosine-inosine-preferring nucleoside ...
-
Kream,Chargaff
, p. 4274 (1952)
-
-
Taylor,Cheng
, p. 9. (1959)
-
Enzymatic deamination of the epigenetic base N-6-methyladenine
Kamat, Siddhesh S.,Fan, Hao,Sauder, J. Michael,Burley, Stephen K.,Shoichet, Brian K.,Sali, Andrej,Raushel, Frank M.
, p. 2080 - 2083 (2011)
Two enzymes of unknown function from the...
Assay of purine nucleoside phosphorylase in erythrocytes by flow-injection analysis with fluorescence detection
Hayashi,Zaitsu,Ohkura
, p. 4574 - 4578 (1987)
-
Localization of purine metabolizing enzymes in bovine brain microvessel endothelial cells: An enzymatic blood-brain barrier for dideoxynucleosides?
Johnson, Mark D.,Andersen, Bradley D.
, p. 1881 - 1886 (1996)
Purpose. The specific activities of the ...
Phloroglucinols Inhibit Chemical Mediators and Xanthine Oxidase, and Protect Cisplatin-Induced Cell Death by Reducing Reactive Oxygen Species in Normal Human Urothelial and Bladder Cancer Cells
Lin, Kai-Wei,Huang, A.-Mei,Tu, Huang-Yao,Weng, Jing-R.U.,Hour, Tzyh-Chyuan,Wei, Bai-Luh,Yang, Shyh-Chyun,Wang, Jih-Pyang,Pu, Yeong-Shiau,Lin, Chun-Nan
, p. 8782 - 8787 (2009)
Phloroglucinols, garcinielliptones HA-HE...
Synthetic method of nitric acid catalyzed hypoxanthine derivative
-
Paragraph 0027-0036, (2021/10/11)
The invention discloses a synthetic meth...
Thermodynamic Reaction Control of Nucleoside Phosphorolysis
Kaspar, Felix,Giessmann, Robert T.,Neubauer, Peter,Wagner, Anke,Gimpel, Matthias
supporting information, p. 867 - 876 (2020/01/24)
Nucleoside analogs represent a class of ...
Synthesis of Adenine Nucleosides by Transglycosylation using Two Sequential Nucleoside Phosphorylase-Based Bioreactors with On-Line Reaction Monitoring by using HPLC
Cattaneo, Giulia,Rabuffetti, Marco,Speranza, Giovanna,Kupfer, Tom,Peters, Benjamin,Massolini, Gabriella,Ubiali, Daniela,Calleri, Enrica
, p. 4614 - 4620 (2017/12/13)
Uridine phosphorylase from Clostridium p...
68-94-0 Process route
-
- 1214-39-7
6-benzyladenine

-
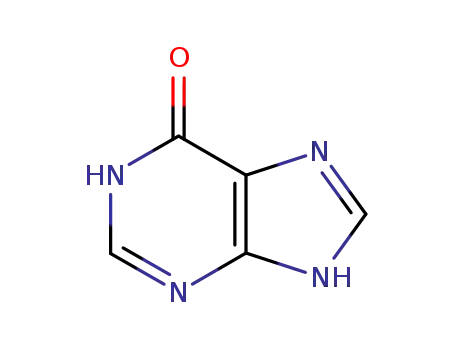
- 68-94-0
hypoxanthine

-
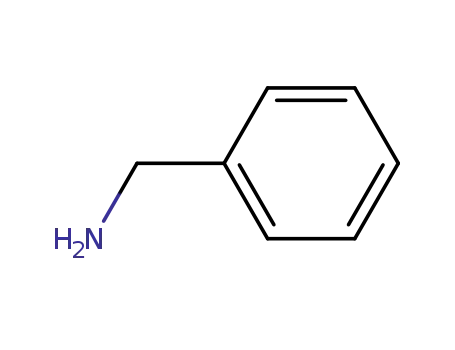
- 100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
With Pseudoalteromonas atlantica T6c cytokinin deaminase Patl2390; water; Reagent/catalyst; Kinetics; Enzymatic reaction;
|
-
- 525-79-1
kinetin

-
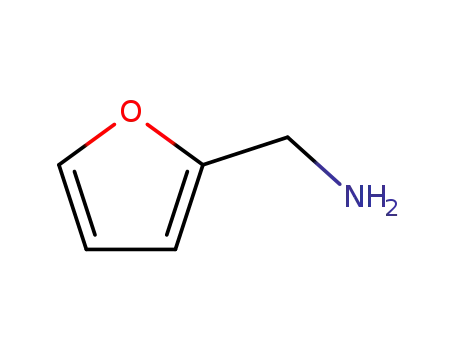
- 617-89-0
furan-2-ylmethanamine

-

- 68-94-0
hypoxanthine
| Conditions | Yield |
|---|---|
|
With Pseudoalteromonas atlantica T6c cytokinin deaminase Patl2390; water; Kinetics; Enzymatic reaction;
|
68-94-0 Upstream products
-
64-18-6
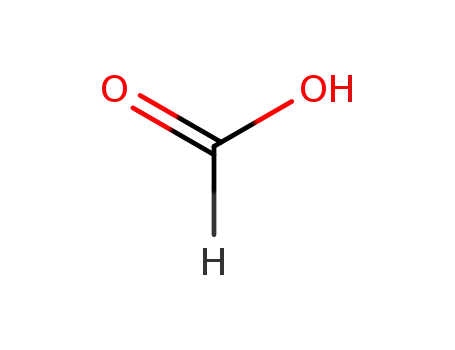
formic acid
-
4316-98-7
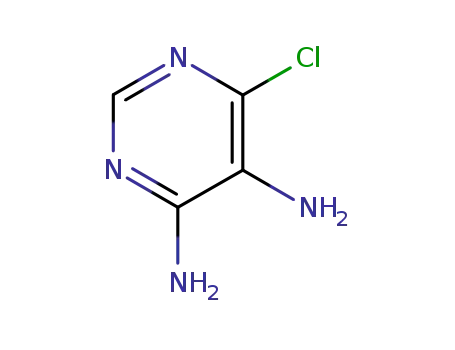
6-chloro-4,5-diaminopyrimidine
-
69-93-2
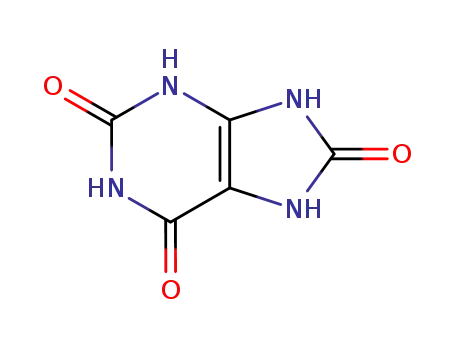
uric Acid
-
77287-34-4
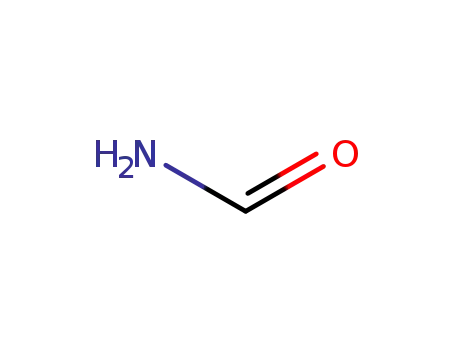
formamide
68-94-0 Downstream products
-
33155-83-8
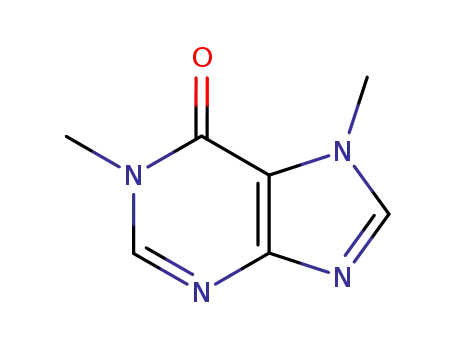
1,7-dimethylhypoxanthine
-
50-44-2
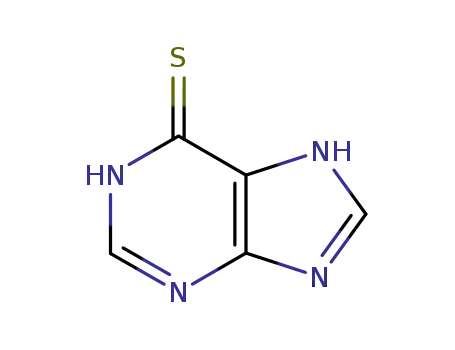
6H-purine-6-thione
-
69-93-2

uric Acid
-
87-42-3
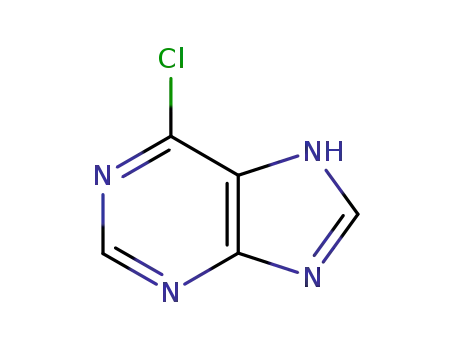
6-chloro-7H-purine
Relevant Products
-
Epinephrine bitartrate
CAS:51-42-3
-
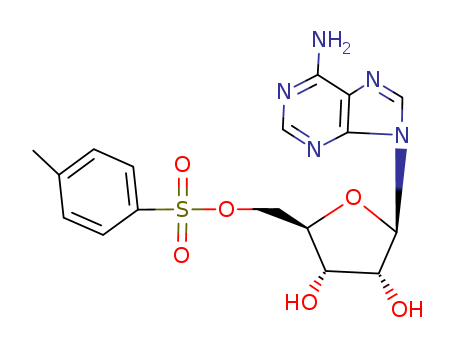
5'-Tosyladenosine
CAS:5135-30-8
-
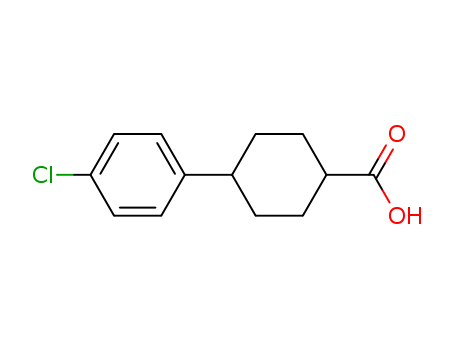
4-(4-Chlorophenyl)cyclohexanecarboxylic acid
CAS:95233-37-7