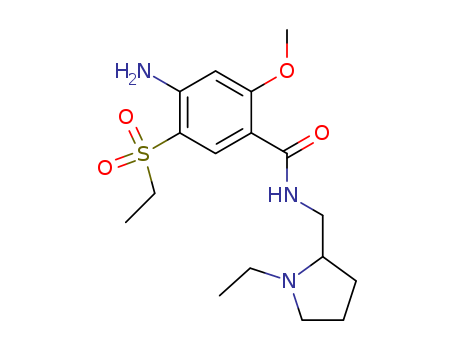
71675-85-9
- Product Name:Amisulpride
- Molecular Formula:C17H27N3O4S
- Purity:99%
- Molecular Weight:369.485
Product Details;
CasNo: 71675-85-9
Molecular Formula: C17H27N3O4S
Appearance: Off-white solid
Factory Sells Quality Manufacturer Supply Amisulpride 71675-85-9 In Stock
- Molecular Formula:C17H27N3O4S
- Molecular Weight:369.485
- Appearance/Colour:Off-white solid
- Vapor Pressure:1.59E-12mmHg at 25°C
- Melting Point:124-128 °C
- Refractive Index:1.545
- Boiling Point:558.9 °C at 760 mmHg
- PKA:13.73±0.46(Predicted)
- Flash Point:291.8 °C
- PSA:110.11000
- Density:1.2 g/cm3
- LogP:3.27590
Amisulpride(Cas 71675-85-9) Usage
|
Description |
Amisulpride i s a n antipsychotic agent structurally related to sulpiride and sultopride. Useful in the treatment of schizophrenia, it is not, however, without EPS liability. |
|
Chemical Properties |
Off-White Solid |
|
Originator |
SESIF (Delagrange) (France) |
|
Uses |
Neuroleptic agent, an analogue of sulpiride. Used as an antipsychotic. Dopamine receptor antagonist |
|
Manufacturing Process |
2-Methoxy-4-amino-5-ethylthiobenzoic acid: 159 g of 2-methoxy-4-amino-5-mercaptobenzoic acid, 355 ml of water and 160 ml of caustic soda solution are placed in a flask fitted with a condenser. The mixture is heated until the solid dissolves, then 123 g of ethyl sulfate is added. The mixture is heated to reflux, treated with 10 ml of 30% caustic soda solution, then heated to reflux for 1 hour. After cooling, 800 ml of water is added and the solution is filtered. The precipitate obtained by adding 100 ml of concentrated hydrochloric acid in the presence of ether is drained, washed with water and dried. 162 g of 2-methoxy-4-amino-5-ethylthiobenzoic acid is obtained (yield=88%). 2-Methoxy-4-amino-5-ethylsulfonylbenzoic acid: 123 g of 2-methoxy-4-amino-5-ethylthiobenzoic acid is dissolved hot in 542 ml of acetic acid. The solution obtained is cooled to 35°C, then 185 ml of hydrogen peroxide is added in small quantities while the temperature is raised to 80°C. The temperature is lowered to 40°C and the mixture is kept at that temperature for some hours and then cooled to 10°C. The precipitate formed is drained, washed with acetic acid and dried, then dissolved in 600 ml of water and 100 ml of 20% ammonia. The precipitate formed by adding 70 ml of concentrated hydrochloric acid is cooled, drained, washed with water and dried. 61.5 g of 2-methoxy-4-amino-5-ethylsulfonylbenzoic acid is obtained (yield 42%, M.P. 95-100°C). 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2- methoxybenzamide: 81 g of 2-methoxy-4-amino-5-ethylsulphonylbenzoic acid and 297 ml of acetone are placed in a flask fitted with an agitator, a thermometer and a dropping funnel, followed by 33 g of triethylamine. The solution is cooled to 0°C, then 30 g of ethyl chloroformate is added drop by drop between 0° and 5°C. When the mixture has been agitated 51 g of 1-ethyl-2- aminomethylpyrrolidine is added drop by drop between 5° and 10°C. The mixture is agitated at 10°C then at ambient temperature. The triethylamine hydrochloride which precipitates is drained, then the acetone is distilled. The residue is dissolved in 600 ml of water in the presence of caustic soda solution. The base crystallizes after seeding and is drained, washed with water and dried. When the crystals have been purified by passing them through hydrochloride and recrystallising them in acetone, 66 g of 4-amino-N-[(1- ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamideis obtained (yield 61%, M.P. 126-127°C). |
|
Brand name |
SOCIAN |
|
Therapeutic Function |
Antipsychotic |
|
Biological Activity |
Potent, selective dopamine D 2 and D 3 receptor antagonist. K i values are 2.8 and 3.2 nM respectively for human D 2 and D 3 and > 1000 nM for human D 1 , D 4 and D 5 receptors. Shows selectivity for presynaptic dopamine autoreceptors at low doses and blocks postsynaptic D 2 /D 3 receptors at higher doses. Preferentially interacts with limbic D 2 -like receptors in vivo . Atypical antipsychotic/antischizophrenic agent with limited extrapyrimidal side effects and a profile distinct from that of haloperidol and remoxipride. |
|
Biochem/physiol Actions |
Amisulpride is a highly selective D2/D3 dopamine receptor antagonist and atypical antipsychotic. |
|
Clinical Use |
Treatment of acute and chronic schizophrenia |
|
Drug interactions |
Potentially hazardous interactions with other drugs Alcohol: may enhance CNS effects of alcohol. Anaesthetics: enhanced hypotensive effect. Analgesics: increased risk of convulsions with tramadol; enhanced hypotensive and sedative effects with opioids; increased risk of ventricular arrhythmias with methadone - avoid. Anti-arrhythmics: increased risk of ventricular arrhythmias with anti-arrhythmics that prolong the QT interval; avoid with amiodarone, disopyramide and procainamide (risk of ventricular arrhythmias). Antibacterials: avoid with erythromycin (increased risk of ventricular arrhythmias). Antidepressants: increased level of tricyclics. Antiepileptics: antagonises anticonvulsant effect. Antihypertensives: increased risk of hypotension. Antimalarials: avoid with artemether/lumefantrine. Antipsychotics: increased risk of ventricular arrhythmias with droperidol, sertindole - avoid. Antivirals: concentration possibly increased by ritonavir. Anxiolytics and hypnotics: increased sedative effects. Atomoxetine: increased risk of ventricular arrhythmias. Beta-blockers: increased risk of ventricular arrhythmias with sotalol. Cytotoxics: increased risk of ventricular arrhythmias with vandetanib - avoid; increased risk of ventricular arrhythmias with arsenic trioxide. Diuretics: increased risk of ventricular arrhythmias due to hypokalaemia. Pentamidine: increased risk of ventricular arrhythmias - avoid. |
|
Metabolism |
Amisulpride is weakly metabolised: two inactive metabolites, accounting for approximately 4% of the dose, have been identified. Amisulpride is eliminated unchanged in the urine. Fifty percent of an intravenous dose is excreted via the urine, of which 90% is eliminated in the first 24 hours. |
|
references |
[1] schoemaker h1, claustre y, fage d, rouquier l, chergui k, curet o, oblin a, gonon f, carter c, benavides j, scatton b. neurochemical characteristics of amisulpride, an atypical dopamine d2/d3 receptor antagonist with both presynaptic and limbic selectivity. j pharmacol exp ther. 1997 jan;280(1):83-97. |
InChI:InChI=1/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21)
71675-85-9 Relevant articles
Preparation method of amisulpride
-
Paragraph 0034-0043, (2021/04/10)
The invention relates to a preparation m...
(S)(-)-amisulpride preparation method
-
Paragraph 0004, (2020/03/06)
The invention relates to an (S)(-)-amisu...
Multicomponent Reductive Cross-Coupling of an Inorganic Sulfur Dioxide Surrogate: Straightforward Construction of Diversely Functionalized Sulfones
Meng, Yingying,Wang, Ming,Jiang, Xuefeng
supporting information, p. 1346 - 1353 (2019/12/11)
Conventionally, sulfones are prepared by...
Aryl alkyl sulfone compound and reducing coupling method for constructing sulfone compounds
-
Paragraph 0362-0366, (2019/12/25)
The invention discloses an aryl alkyl su...
71675-85-9 Process route
-
-
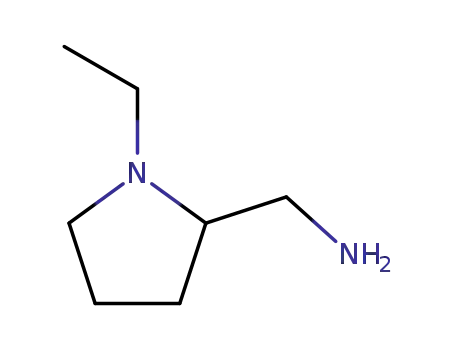
26116-12-1
1-ethyl-2-pyrrolidinemethanamine

-
-
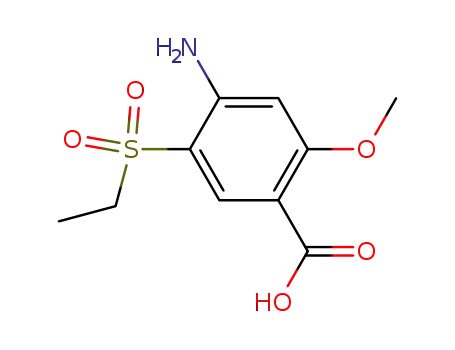
71675-87-1
2-methoxy-4-amino-5-ethylsulphonyl benzoic acid

-
-
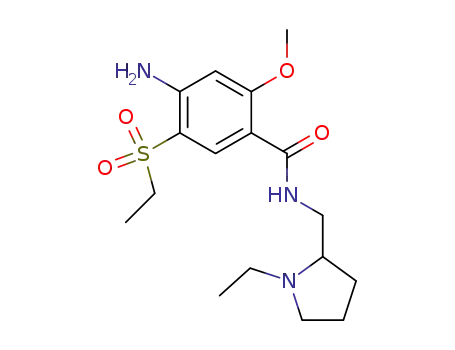
71675-85-9
amisulpride
| Conditions | Yield |
|---|---|
|
2-methoxy-4-amino-5-ethylsulphonyl benzoic acid;
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 10 - 20 ℃;
for 1.5h;
1-ethyl-2-pyrrolidinemethanamine;
With
triethylamine;
In
acetonitrile;
at 80 ℃;
for 2h;
|
86.8% |
|
With
C36H24B4N2O3;
In
toluene;
for 14h;
chemoselective reaction;
Molecular sieve;
Reflux;
|
76% |
|
With
chloroformic acid ethyl ester; triethylamine;
In
acetone;
at 0 - 30 ℃;
|
70% |
-

-
26116-12-1
1-ethyl-2-pyrrolidinemethanamine

-
-
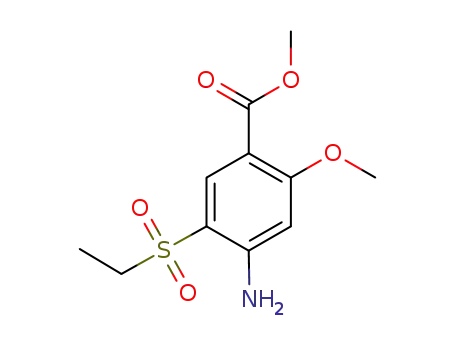
80036-89-1
methyl 4-amino-5-(ethylsulfonyl)-2-methoxybenzoate

-

-
71675-85-9
amisulpride
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate;
In
tert-butyl alcohol;
at 83 ℃;
for 20h;
Reagent/catalyst;
Solvent;
Temperature;
|
96.5% |
71675-85-9 Upstream products
-
26116-12-1

1-ethyl-2-pyrrolidinemethanamine
-
541-41-3
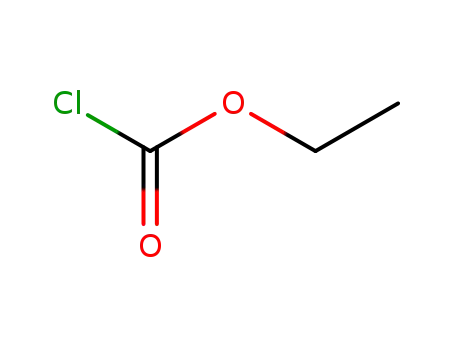
chloroformic acid ethyl ester
-
71675-87-1

2-methoxy-4-amino-5-ethylsulphonyl benzoic acid
-
27492-84-8
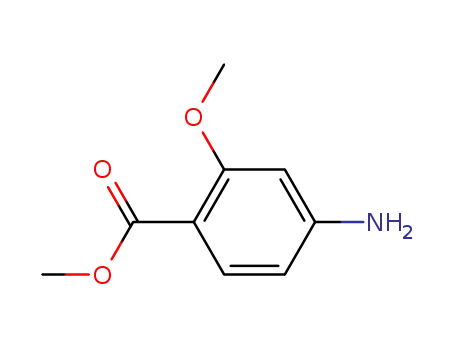
Methyl 4-amino-2-methoxybenzoate
71675-85-9 Downstream products
-
72005-57-3
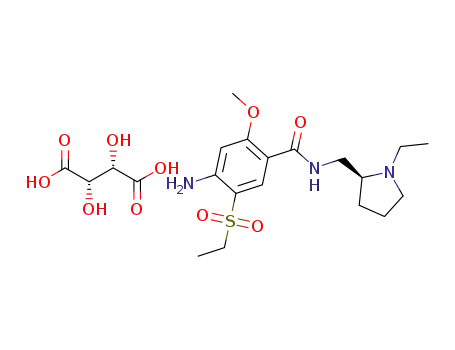
(S)-(-)-4-amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide D-tartrate
Relevant Products
-
Benzathine Cloxacillin
CAS:23736-58-5
-
2'-Deoxyadenosine
CAS:958-09-8
-
chlorophyllin copper complex sodium salt
CAS:65963-40-8